Abstract
Purpose
The neutrophil-to-lymphocyte ratio (NLR) has been reported to relate to the prognosis of various cancers. The aim of this study was to elucidate the efficiency of pre-treatment NLR as a predictor of outcomes of brain metastasis underwent gamma knife radiosurgery (GKRS).
Methods
We analyzed 195 cases with brain metastasis underwent GKRS at our institution between January 2015 and April 2018. Patients’ clinical and radiographic data were collected.
Results
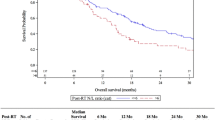
We identified 458 brain metastases in 195 patients. Optimal dichotomous cutoff values of NLR determined by receiver operating characteristic analysis for local control, distant control and overall survival (OS) were 2.48, 2.74 and 3.13, respectively. The actuarial local control rates of patients with high NLR were 87.4% at 6 months and 76.1% at 12 months, whereas that of patients with low NLR were 94.2% at 6 months and 88.3% at 12 months (P = 0.001). The actuarial distant control rates of patients with high NLR were 31.4% at 6 months and 18.9% at 12 months, whereas that of patients with low NLR were 58.5% at 6 months and 31.3% at 12 months (P = 0.001). The median OS of patients with high and low NLR were 10.0 months and 14.5 months, respectively (P = 0.001). Multivariate analysis demonstrates that high NLR independently predicts local failure (hazard ratio [HR], 2.281; P = 0.003), distant brain failure (HR 1.775; P = 0.002) and poorer overall survival (HR 1.494; P = 0.034).
Conclusion
The pre-SRS NLR, a systemic inflammatory marker for treatment response, inversely predicts local control, distant control and OS in patients with brain metastasis.


Similar content being viewed by others
References
Aoyama H, Shirato H, Tago M et al (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491. https://doi.org/10.1001/jama.295.21.2483
Cagney DN, Martin AM, Catalano PJ et al (2017) Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19:1511–1521. https://doi.org/10.1093/neuonc/nox077
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316:401–409. https://doi.org/10.1001/jama.2016.9839
Doron H, Pukrop T, Erez N (2019) A blazing landscape: neuroinflammation shapes brain metastasis. Cancer Res 79:423–436. https://doi.org/10.1158/0008-5472.CAN-18-1805
Owonikoko TK, Arbiser J, Zelnak A et al (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11:203–222. https://doi.org/10.1038/nrclinonc.2014.25
Scoccianti S, Ricardi U (2012) Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol 102:168–179. https://doi.org/10.1016/j.radonc.2011.08.041
Sperduto PW, Kased N, Roberge D et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425. https://doi.org/10.1200/JCO.2011.38.0527
Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK (2011) The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol 8:344–356. https://doi.org/10.1038/nrclinonc.2011.58
Patchell RA, Tibbs PA, Regine WF et al (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489. https://doi.org/10.1001/jama.280.17.1485
Gempt J, Gerhardt J, Toth V et al (2013) Postoperative ischemic changes following brain metastasis resection as measured by diffusion-weighted magnetic resonance imaging. J Neurosurg 119:1395–1400. https://doi.org/10.3171/2013.9.JNS13596
Baschnagel AM, Meyer KD, Chen PY et al (2013) Tumor volume as a predictor of survival and local control in patients with brain metastases treated with Gamma Knife surgery. J Neurosurg 119:1139–1144. https://doi.org/10.3171/2013.7.JNS13431
Jani A, Rozenblat T, Yaeh AM et al (2015) The energy index does not affect local control of brain metastases treated by gamma knife stereotactic radiosurgery. Neurosurgery 77:119–125. https://doi.org/10.1227/NEU.0000000000000750 (discussion 25)
Minniti G, Scaringi C, Paolini S et al (2016) Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (%3e 2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 95:1142–1148. https://doi.org/10.1016/j.ijrobp.2016.03.013
Oermann EK, Kress MA, Todd JV et al (2013) The impact of radiosurgery fractionation and tumor radiobiology on the local control of brain metastases. J Neurosurg 119:1131–1138. https://doi.org/10.3171/2013.8.JNS122177
Gaspar L, Scott C, Rotman M et al (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751. https://doi.org/10.1016/s0360-3016(96)00619-0
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514. https://doi.org/10.1016/j.ijrobp.2007.06.074
Mitsuya K, Nakasu Y, Kurakane T, Hayashi N, Harada H, Nozaki K (2017) Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of worse survival after resection in patients with brain metastasis. J Neurosurg 127:433–437. https://doi.org/10.3171/2016.8.JNS16899
Cannon NA, Meyer J, Iyengar P et al (2015) Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. Journal of Thoracic Oncology 10:280–285. https://doi.org/10.1097/Jto.0000000000000399
Lopes M, Carvalho B, Vaz R, Linhares P (2018) Influence of neutrophil-lymphocyte ratio in prognosis of glioblastoma multiforme. J Neurooncol 136:173–180. https://doi.org/10.1007/s11060-017-2641-3
Koh CH, Bhoo-Pathy N, Ng KL et al (2015) Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer 113:150–158. https://doi.org/10.1038/bjc.2015.183
Grenader T, Waddell T, Peckitt C et al (2016) Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 27:687–692. https://doi.org/10.1093/annonc/mdw012
Gemenetzis G, Bagante F, Griffin JF et al (2017) Neutrophil-to-lymphocyte ratio is a predictive marker for invasive malignancy in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 266:339–345. https://doi.org/10.1097/Sla.0000000000001988
Kim JH, Lee JY, Kim HK et al (2017) Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol 23:505–515. https://doi.org/10.3748/wjg.v23.i3.505
Koh YW, Choi JH, Ahn MS, Choi YW, Lee HW (2016) Baseline neutrophil-lymphocyte ratio is associated with baseline and subsequent presence of brain metastases in advanced non-small-cell lung cancer. Sci Rep 6:38585. https://doi.org/10.1038/srep38585
Chowdhary M, Switchenko JM, Press RH et al (2018) Post-treatment neutrophil-to-lymphocyte ratio predicts for overall survival in brain metastases treated with stereotactic radiosurgery. J Neurooncol 139:689–697. https://doi.org/10.1007/s11060-018-2914-5
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Sneed PK, Mendez J, Vemer-van den Hoek JG et al (2015) Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg 123:373–386. https://doi.org/10.3171/2014.10.JNS141610
Ly D, Bagshaw HP, Anker CJ et al (2015) Local control after stereotactic radiosurgery for brain metastases in patients with melanoma with and without BRAF mutation and treatment. J Neurosurg 123:395–401. https://doi.org/10.3171/2014.9.JNS141425
Grandhi R, Kondziolka D, Panczykowski D et al (2012) Stereotactic radiosurgery using the Leksell Gamma Knife Perfexion unit in the management of patients with 10 or more brain metastases. J Neurosurg 117:237–245. https://doi.org/10.3171/2012.4.JNS11870
Romano KD, Trifiletti DM, Garda A et al (2017) Choosing a prescription isodose in stereotactic radiosurgery for brain metastases: implications for local control. World Neurosurg 98(761–7):e1. https://doi.org/10.1016/j.wneu.2016.11.038
Black PM, Johnson MD (2004) Surgical resection for patients with solid brain metastases: current status. J Neurooncol 69:119–124. https://doi.org/10.1023/b:neon.0000041875.09048.e7
Arita H, Narita Y, Miyakita Y, Ohno M, Sumi M, Shibui S (2014) Risk factors for early death after surgery in patients with brain metastases: reevaluation of the indications for and role of surgery. J Neurooncol 116:145–152. https://doi.org/10.1007/s11060-013-1273-5
Linskey ME, Andrews DW, Asher AL et al (2010) The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 96:45–68. https://doi.org/10.1007/s11060-009-0073-4
Yamamoto M, Serizawa T, Shuto T et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. https://doi.org/10.1016/s1470-2045(14)70061-0
Rava P, Leonard K, Sioshansi S et al (2013) Survival among patients with 10 or more brain metastases treated with stereotactic radiosurgery. J Neurosurg 119:457–462. https://doi.org/10.3171/2013.4.JNS121751
Chang EL, Wefel JS, Hess KR et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/s1470-2045(09)70263-3
Wolf A, Kvint S, Chachoua A et al (2018) Toward the complete control of brain metastases using surveillance screening and stereotactic radiosurgery. J Neurosurg 128:23–31. https://doi.org/10.3171/2016.10.JNS161036
Sperduto PW, Chao ST, Sneed PK et al (2010) Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 77:655–661. https://doi.org/10.1016/j.ijrobp.2009.08.025
Doin H, Nakamatsu K, Anami S et al (2019) Neutrophil-to-lymphocyte ratio predicts survival after whole-brain radiotherapy in non-small cell lung cancer. In Vivo 33:195–201. https://doi.org/10.21873/invivo.11459
Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16:431–446. https://doi.org/10.1038/nrc.2016.52
Charles KA, Kulbe H, Soper R et al (2009) The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Investig 119:3011–3023. https://doi.org/10.1172/JCI39065
Di Mitri D, Toso A, Chen JJ et al (2014) Tumour-infiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature 515:134–137. https://doi.org/10.1038/nature13638
Houghton AM, Rzymkiewicz DM, Ji H et al (2010) Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 16:219–223. https://doi.org/10.1038/nm.2084
Shojaei F, Wu X, Zhong C et al (2007) Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450:825–831. https://doi.org/10.1038/nature06348
Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP (2014) Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia 16:771–788. https://doi.org/10.1016/j.neo.2014.08.013
Spicer JD, McDonald B, Cools-Lartigue JJ et al (2012) Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 72:3919–3927. https://doi.org/10.1158/0008-5472.CAN-11-2393
Cools-Lartigue J, Spicer J, McDonald B et al (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Investig 123:3346–3458. https://doi.org/10.1172/JCI67484
Martinez-Lostao L, Anel A, Pardo J (2015) How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res 21:5047–5056. https://doi.org/10.1158/1078-0432.CCR-15-0685
Louveau A, Smirnov I, Keyes TJ et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. https://doi.org/10.1038/nature14432
Neal MT, Chan MD, Lucas JT Jr et al (2014) Predictors of survival, neurologic death, local failure, and distant failure after gamma knife radiosurgery for melanoma brain metastases. World Neurosurg 82:1250–1255. https://doi.org/10.1016/j.wneu.2013.02.025
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individuals included in the study, whenever possible, taking into account the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Hu, Y., Chen, W. et al. Pre-stereotactic radiosurgery neutrophil-to-lymphocyte ratio is a predictor of the prognosis for brain metastases. J Neurooncol 147, 691–700 (2020). https://doi.org/10.1007/s11060-020-03477-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03477-w