Abstract
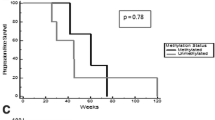
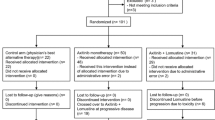
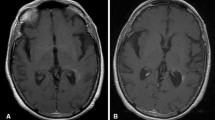
Prognosis of recurrent glioblastoma (GBM) is poor with 6-month progression-free survival (PFS6) ranging from 9 to 48% depending on the treatment regimen and use of anti-angiogenic therapies. We sought to study vorinostat (VOR), a histone deacetylase inhibitor, in combination with bevacizumab (BEV) and daily metronomic temozolomide (TMZ) in a Phase I/II trial in recurrent high-grade gliomas (HGGs). This was a Phase I/II open-label, single-arm study in recurrent HGG patients. Phase I primary endpoint was to determine the maximum tolerated dose (MTD) of VOR with BEV and daily TMZ. Phase II primary endpoint was PFS6. Regimen was BEV 10 mg/kg iv every 2 weeks, TMZ 50 mg/m2 po daily, and VOR 200 or 400 mg po alternating 7 days on then 7 days off throughout a 28-day cycle. Phase I portion enrolled nine subjects with three receiving VOR 200 mg and 6 receiving VOR 400 mg. With no dose-limiting toxicities (DLTs) at 200 mg and one DLT (thrombocytopenia, Grade 3) at 400 mg, the MTD was 400 mg. Phase II portion enrolled 39 GBM subjects, and PFS6 was 53.8% (95% CI 37.2–67.9%). Of note, 14 subjects had received prior BEV and all had received prior 5-day TMZ. Combination therapy with VOR, BEV, and daily TMZ was well tolerated and safe. While PFS6 was not statistically improved beyond historical controls, it is important to note that this was a heavily pretreated GBM population and further consideration is warranted in a less pretreated group.

Similar content being viewed by others
Abbreviations
- HGG:
-
High grade glioma
- GBM:
-
Glioblastoma
- TMZ:
-
Temozolomide
- BEV:
-
Bevacizumab
- VOR:
-
Vorinostat
- MRI:
-
Magnetic resonance imaging
- RANO:
-
Response Assessment in Neuro-Oncology
- CI:
-
Confidence interval
- PFS:
-
Progression-free survival
- PFS6:
-
6-Month progression-free survival
- OS:
-
Overall survival
- PR:
-
Partial response
- CR:
-
Complete response
- DVT:
-
Deep venous thrombosis
- PE:
-
Pulmonary embolus
- MGMT:
-
Methyl guanine methyl transferase
- KPS:
-
Karnofsky performance status
- MTD:
-
Maximum tolerated dose
- DLT:
-
Dose-limiting toxicity
- HDAC:
-
Histone deacetylases
- CBC:
-
Complete blood count
- CMP:
-
Comprehensive metabolic panel
- ULN:
-
Upper limit of normal
References
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359–364
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Weller M, Cloughesy T, Perry JR, Wick W (2013) Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro Oncol 15:4–27
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14:1131–1138
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE 2nd, Bailey L, Peters KB, Friedman HS, Vredenburgh JJ (2012) Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 118:1302–1312
Galanis E, Anderson SK, Lafky JM, Uhm JH, Giannini C, Kumar SK, Kimlinger TK, Northfelt DW, Flynn PJ, Jaeckle KA, Kaufmann TJ, Buckner JC (2013) Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): a north central cancer treatment group trial. Clin Cancer Res 19:4816–4823
Lassen U, Sorensen M, Gaziel TB, Hasselbalch B, Poulsen HS (2013) Phase II study of bevacizumab and temsirolimus combination therapy for recurrent glioblastoma multiforme. Anticancer Res 33:1657–1660
Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, Herndon JE 2nd, Bulusu A, Threatt S, Friedman AH, Vredenburgh JJ, Friedman HS (2012) Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol 107:155–164
Reardon DA, Desjardins A, Vredenburgh JJ, Gururangan S, Sampson JH, Sathornsumetee S, McLendon RE, Herndon JE 2nd, Marcello JE, Norfleet J, Friedman AH, Bigner DD, Friedman HS (2009) Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer 101:1986–1994
Sathornsumetee S, Desjardins A, Vredenburgh JJ, McLendon RE, Marcello J, Herndon JE, Mathe A, Hamilton M, Rich JN, Norfleet JA, Gururangan S, Friedman HS, Reardon DA (2010) Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol 12:1300–1310
Soffietti R, Trevisan E, Bertero L, Cassoni P, Morra I, Fabrini MG, Pasqualetti F, Lolli I, Castiglione A, Ciccone G, Ruda R (2014) Bevacizumab and fotemustine for recurrent glioblastoma: a phase II study of AINO (Italian Association of Neuro-Oncology). J Neurooncol 116:533–541
Verhoeff JJ, Lavini C, van Linde ME, Stalpers LJ, Majoie CB, Reijneveld JC, van Furth WR, Richel DJ (2010) Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol 21:1723–1727
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Duvic M, Vu J (2007) Vorinostat: a new oral histone deacetylase inhibitor approved for cutaneous T-cell lymphoma. Expert Opin Investig Drugs 16:1111–1120
Siegel D, Hussein M, Belani C, Robert F, Galanis E, Richon VM, Garcia-Vargas J, Sanz-Rodriguez C, Rizvi S (2009) Vorinostat in solid and hematologic malignancies. J Hematol Oncol 2:31
Galanis E, Jaeckle KA, Maurer MJ, Reid JM, Ames MM, Hardwick JS, Reilly JF, Loboda A, Nebozhyn M, Fantin VR, Richon VM, Scheithauer B, Giannini C, Flynn PJ, Moore DF Jr, Zwiebel J, Buckner JC (2009) Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol 27:2052–2058
Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN (2004) Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem 92:223–237
Rundall BK, Denlinger CE, Jones DR (2005) Suberoylanilide hydroxamic acid combined with gemcitabine enhances apoptosis in non-small cell lung cancer. Surgery 138:360–367
Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, Hoshino K, Quintas-Cardama A, Richon VM, Garcia-Manero G (2006) Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood 108:1174–1182
Dasmahapatra G, Yerram N, Dai Y, Dent P, Grant S (2007) Synergistic interactions between vorinostat and sorafenib in chronic myelogenous leukemia cells involve Mcl-1 and p21CIP1 down-regulation. Clin Cancer Res 13:4280–4290
Shen J, Huang C, Jiang L, Gao F, Wang Z, Zhang Y, Bai J, Zhou H, Chen Q (2007) Enhancement of cisplatin induced apoptosis by suberoylanilide hydroxamic acid in human oral squamous cell carcinoma cell lines. Biochem Pharmacol 73:1901–1909
Arnold NB, Arkus N, Gunn J, Korc M (2007) The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces growth inhibition and enhances gemcitabine-induced cell death in pancreatic cancer. Clin Cancer Res 13:18–26
Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H (2009) Enhancement of cisplatin cytotoxicity by SAHA involves endoplasmic reticulum stress-mediated apoptosis in oral squamous cell carcinoma cells. Cancer Chemother Pharmacol 64:1115–1122
Rikiishi H, Shinohara F, Sato T, Sato Y, Suzuki M, Echigo S (2007) Chemosensitization of oral squamous cell carcinoma cells to cisplatin by histone deacetylase inhibitor, suberoylanilide hydroxamic acid. Int J Oncol 30:1181–1188
Sarcar B, Kahali S, Chinnaiyan P (2010) Vorinostat enhances the cytotoxic effects of the topoisomerase I inhibitor SN38 in glioblastoma cell lines. J Neurooncol 99:201–207
Bangert A, Hacker S, Cristofanon S, Debatin KM, Fulda S (2011) Chemosensitization of glioblastoma cells by the histone deacetylase inhibitor MS275. Anticancer Drugs 22:494–499
Shao CJ, Wu MW, Chen FR, Li C, Xia YF, Chen ZP (2012) Histone deacetylase inhibitor, 2-propylpentanoic acid, increases the chemosensitivity and radiosensitivity of human glioma cell lines in vitro. Chin Med J (Engl) 125:4338–4343
Sampson VB, Vetter NS, Kamara DF, Collier AB, Gresh RC, Kolb EA (2015) Vorinostat enhances cytotoxicity of SN-38 and temozolomide in ewing sarcoma cells and activates STAT3/AKT/MAPK pathways. PLoS ONE 10:e0142704
Lee EQ, Puduvalli VK, Reid JM, Kuhn JG, Lamborn KR, Cloughesy TF, Chang SM, Drappatz J, Yung WK, Gilbert MR, Robins HI, Lieberman FS, Lassman AB, McGovern RM, Xu J, Desideri S, Ye X, Ames MM, Espinoza-Delgado I, Prados MD, Wen PY (2012) Phase I study of vorinostat in combination with temozolomide in patients with high-grade gliomas: North American Brain Tumor Consortium Study 04-03. Clin Cancer Res 18:6032–6039
Grant S, Easley C, Kirkpatrick P (2007) Vorinostat. Nat Rev Drug Discov 6:21–22
Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, Blacher S, Verdin E, Foidart JM, Nusgens BV, Castronovo V (2002) Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene 21:427–436
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol 28:1963–1972
Chinnaiyan P, Chowdhary S, Potthast L, Prabhu A, Tsai YY, Sarcar B, Kahali S, Brem S, Yu HM, Rojiani A, Murtagh R, Pan E (2012) Phase I trial of vorinostat combined with bevacizumab and CPT-11 in recurrent glioblastoma. Neuro Oncol 14:93–100
Weller M, Tabatabai G, Kastner B, Felsberg J, Steinbach JP, Wick A, Schnell O, Hau P, Herrlinger U, Sabel MC, Wirsching HG, Ketter R, Bahr O, Platten M, Tonn JC, Schlegel U, Marosi C, Goldbrunner R, Stupp R, Homicsko K, Pichler J, Nikkhah G, Meixensberger J, Vajkoczy P, Kollias S, Husing J, Reifenberger G, Wick W, Group DS (2015) MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR Trial. Clin Cancer Res 21:2057–2064
Ghiaseddin A, Reardon DA, Massey W, Mannerino A, Lipp ES, Herndon JE, McSherry F, Desjardins A, Randazzo DM, Vlahovic G, Friedman HS, Peters KB (2015) Phase II study of bevacizumab and vorinostat for recurrent glioblastoma. J Clin Oncol. https://doi.org/10.1200/jco.2015.33.15_suppl.2034
Puduvalli VK, Wu J, Yuan Y, Armstrong TS, Groves MD, Raizer JJ, Giglio P, Colman H, Peereboom DM, Walbert T, Avgeropoulos N, Iwamoto FM, Chamberlain MC, Paleologos N, Fink K, Merrell R, Yung WK, Gilbert M (2015) Brain Tumor Trials Collaborative Bayesian adaptive radnomized phase II trial of bevacizumab plus vorinostat versus bevacizumab alone in adults with recurrent glioblastoma (BTTC-1102). J Clin Oncol. https://doi.org/10.1200/jco.2015.33.15_suppl.2012
Acknowledgements
We would like to thank Mrs. Kelly Seagroves for assisting with preparation of this manuscript. This work was supported by Merck Sharp & Dohme Corp and Genentech, Inc.
Funding
VOR was provided by Merck Sharp & Dohme Corp. BEV was provided by Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AD received financial compensation from Genentech/Roche for Advisory Board participation. HSF received financial compensation from Genentech/Roche for Speakers Bureau participation and consultation. DAR received financial compensation from Genentech/Roche and Merck Advisory Board participation. The rest of the authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Peters, K.B., Lipp, E.S., Miller, E. et al. Phase I/II trial of vorinostat, bevacizumab, and daily temozolomide for recurrent malignant gliomas. J Neurooncol 137, 349–356 (2018). https://doi.org/10.1007/s11060-017-2724-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2724-1