Abstract
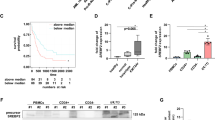
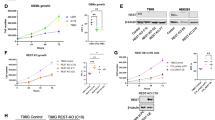
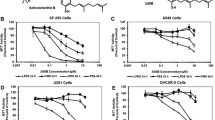
Glioblastoma (GBM) is the most common primary brain tumor. Genetic mutations may reprogram the metabolism of neoplastic cells. Particularly, alterations in cholesterol and fatty acid biosynthetic pathways may favor biomass synthesis and resistance to therapy. Therefore, compounds that interfere with those pathways, such as phytol (PHY) and retinol (RET), may be appropriate for cytotoxic approaches. We tested the effect of PHY or RET on the viability of human GBM cell lines (U87MG, A172 and T98G). Since the compounds showed a dose-dependent cytotoxic effect, additional analyses were performed with IC50 values. Transcriptome analyses of A172 cells treated with PHY IC50 or RET IC50 revealed down-regulated genes involved in cholesterol and/or fatty acid biosynthetic pathways. Thus, we investigated the expression of proteins required for cholesterol and/or fatty acid synthesis after treating all lineages with PHY IC50 or RET IC50 and comparing them with controls. Sterol regulatory element-binding protein 1 (SREBP-1) expression was reduced by PHY in U87 and T98G cells. However, fatty acid synthase (FAS) protein expression, which is regulated by SREBP-1, was down-regulated in all lineages after both treatments. Moreover, farnesyl-diphosphate farnesyltransferase (FDFT1) levels, a protein associated with cholesterol synthesis, were reduced in all lineages by PHY and in U87MG and A172 cells by RET. Our results suggest that SREBP-1, FAS and FDFT1 are potential target(s) for future in vivo approaches against GBM and support the use of inhibitors of their synthesis, including PHY and RET, for such approaches.




Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Yoshino A, Ogino A, Yachi K et al (2010) Gene expression profiling predicts response to temozolomide in malignant gliomas. Int J Oncol 36:1367–1377
Venneti S, Mischel PS (2015) Metabolic reprogramming in brain cancer: A coordinated effort. Brain Pathol 25:753–754. https://doi.org/10.1111/bpa.12308
Pejin B, Kojic V, Bogdanovic G (2014) An insight into the cytotoxic activity of phytol at in vitro conditions. Nat Prod Res 28:2053–2056. https://doi.org/10.1080/14786419.2014.921686
An Y, Zhang D-D, Yu H-L et al (2017) 27-Hydroxycholesterol regulates cholesterol synthesis and transport in C6 glioma cells. Neurotoxicology. https://doi.org/10.1016/j.neuro.2017.02.001
Eberlé D, Hegarty B, Bossard P et al (2004) SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86:839–848. https://doi.org/10.1016/j.biochi.2004.09.018
Ferré P, Foufelle F (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diab Obes Metab 12:83–92. https://doi.org/10.1111/j.1463-1326.2010.01275.x
Jayakumar A (1994) Isolation and chromosomal mapping of genomic clones encoding the human fatty acid synthase gene. Genomics 23:420–424. https://doi.org/10.1006/geno.1994.1518
Norrmén C, Figlia G, Lebrun-Julien F et al (2014) mTORC1 controls PNS myelination along the mTORC1-RXRγ-SREBP-lipid biosynthesis axis in schwann cells. Cell Rep 9:646–660. https://doi.org/10.1016/j.celrep.2014.09.001
Luo D, Xiao H, Dong J et al (2017) B7-H3 regulates lipid metabolism of lung cancer through SREBP1-mediated expression of FASN. Biochem Biophys Res Commun 482:1246–1251. https://doi.org/10.1016/j.bbrc.2016.12.021
van Deijk A-LF, Camargo N, Timmerman J et al (2017) Astrocyte lipid metabolism is critical for synapse development and function in vivo. Glia 65:670–682. https://doi.org/10.1002/glia.23120
Tansey T (2000) Structure and regulation of mammalian squalene synthase. Biochim Biophys Acta—Mol Cell Biol Lipids 1529:49–62. https://doi.org/10.1016/S1388-1981(00)00137-2
Crick DC, Suders J, Kluthe CM et al (1995) Selective inhibition of cholesterol biosynthesis in brain cells by squalestatin 1. J Neurochem 65:1365–1373
Kitareewan S, Burka LT, Tomer KB et al (1996) Phytol metabolites are circulating dietary factors that activate the nuclear receptor RXR. Mol Biol Cell 7:1153–1166
Lemotte PK, Keidel S, Apfel CM (1996) Phytanic acid is a retinoid X receptor ligand. Eur J Biochem 236:328–333
Zhao S, Li R, Li Y et al (2012) Roles of vitamin A status and retinoids in glucose and fatty acid metabolism. Biochem Cell Biol 90:142–152. https://doi.org/10.1139/o11-079
Tang K, Cao L, Fan S et al (2008) Effect of all-trans-retinoic acid on C6 glioma cell proliferation and differentiation. Zhong Nan Da Xue Xue Bao Yi Xue Ban 33:892–897
Karsy M, Albert L, Murali R, Jhanwar-Uniyal M (2014) The impact of arsenic trioxide and all-trans retinoic acid on p53 R273H-codon mutant glioblastoma. Tumour Biol 35:4567–4580. https://doi.org/10.1007/s13277-013-1601-6
Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69:581–593
Ignarro RS, Facchini G, Vieira AS et al (2016) Sulfasalazine intensifies temozolomide cytotoxicity in human glioblastoma cells. Mol Cell Biochem 418:167–178. https://doi.org/10.1007/s11010-016-2742-x
Mitchener JS, Shelburne JD, Bradford WD, Hawkins HK (1976) Cellular autophagocytosis induced by deprivation of serum and amino acids in HeLa cells. Am J Pathol 83:485–492
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Vieira AS, Rezende ACS, Grigoletto J et al (2009) Ciliary neurotrophic factor infused intracerebroventricularly shows reduced catabolic effects when linked to the TAT protein transduction domain. J Neurochem 110:1557–1566. https://doi.org/10.1111/j.1471-4159.2009.06259.x
Romero-Calvo I, Ocón B, Martínez-Moya P et al (2010) Reversible Ponceau staining as a loading control alternative to actin in western blots. Anal Biochem 401:318–320. https://doi.org/10.1016/j.ab.2010.02.036
Ignarro RS, Vieira AS, Sartori CR et al (2013) JAK2 inhibition is neuroprotective and reduces astrogliosis after quinolinic acid striatal lesion in adult mice. J Chem Neuroanat 48–49:14–22. https://doi.org/10.1016/j.jchemneu.2013.02.005
Guo D, Prins RM, Dang J et al (2009) EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal 2:ra82–ra82. https://doi.org/10.1126/scisignal.2000446
Guo D, Bell EEH, Chakravarti A (2013) Lipid metabolism emerges as a promising target for malignant glioma therapy. CNS Oncol 2:289–299. https://doi.org/10.2217/cns.13.20.Lipid
Geng F, Cheng X, Wu X et al (2016) Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin Cancer Res 22:5337–5348. https://doi.org/10.1158/1078-0432.CCR-15-2973
Venneti S, Thompson CB (2017) Metabolic reprogramming in brain tumors. Annu Rev Pathol Mech Dis 12:515–545. https://doi.org/10.1146/annurev-pathol-012615-044329
Allen M, Bjerke M, Edlund H et al (2016) Origin of the U87MG glioma cell line: good news and bad news. Sci Transl Med 8:354re3–354re3. https://doi.org/10.1126/scitranslmed.aaf6853
Kiseleva LN, Kartashev AV, Vartanyan NL et al (2016) A172 and T98G cell lines characteristics. Cell Tissue Biol 10:341–348. https://doi.org/10.1134/S1990519X16050072
Tao B-B, He H, Shi X et al (2013) Up-regulation of USP2a and FASN in gliomas correlates strongly with glioma grade. J Clin Neurosci 20:717–720. https://doi.org/10.1016/j.jocn.2012.03.050
Yasumoto Y, Miyazaki H, Vaidyan LK et al (2016) Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS ONE 11:e0147717. https://doi.org/10.1371/journal.pone.0147717
Flavin R, Peluso S, Nguyen PL, Loda M (2010) Fatty acid synthase as a potential therapeutic target in cancer. Futur Oncol 6:551–562. https://doi.org/10.2217/fon.10.11
Fukuma Y, Matsui H, Koike H et al (2012) Role of squalene synthase in prostate cancer risk and the biological aggressiveness of human prostate cancer. Prostate Cancer Prostatic Dis 15:339–345. https://doi.org/10.1038/pcan.2012.14
Zhang F, Dai X, Wang Y (2012) 5-Aza-2′-deoxycytidine induced growth inhibition of leukemia cells through modulating endogenous cholesterol biosynthesis. Mol Cell Proteomics 11:M111.016915–M111.016915. https://doi.org/10.1074/mcp.M111.016915
Yang Y-F, Jan Y-H, Liu Y-P et al (2014) Squalene synthase induces tumor necrosis factor receptor 1 enrichment in lipid rafts to promote lung cancer metastasis. Am J Respir Crit Care Med 190:675–687. https://doi.org/10.1164/rccm.201404-0714OC
Jayashree BS, Nigam S, Pai A et al (2015) Targets in anticancer research—A review. Indian J Exp Biol 53:489–507
Bendich A, Langseth L (1989) Safety of vitamin A. Am J Clin Nutr 49:358–371
Funding
This work was supported by grants from FAPESP (2011/50400-0; 2013/02618-1; 2013/07559-3) and FAEPEX/UNICAMP (379/13; 554/14; 621/14).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2017_2672_MOESM1_ESM.xls
Online Resource 1. Gene expression data in human glioblastoma A172 cells after 72 hours of treatment with PHY IC50 or RET IC50. Columns in Tables 1-2 show gene symbols, log2fold changes relative to DMEM with 0.1% DMSO treated cells, p-values and adjusted p-values. Tables 3-6 contain enriched pathways for up- and down-regulated genes for each group. (XLS 4698 KB)
Rights and permissions
About this article
Cite this article
Facchini, G., Ignarro, R.S., Rodrigues-Silva, E. et al. Toxic effects of phytol and retinol on human glioblastoma cells are associated with modulation of cholesterol and fatty acid biosynthetic pathways. J Neurooncol 136, 435–443 (2018). https://doi.org/10.1007/s11060-017-2672-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2672-9