Abstract
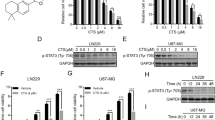
Temozolomide (TMZ) is an alkylating agent used to treat glioblastoma. This tumor type synthesizes the antioxidant glutathione through system X −c , which is inhibited by sulfasalazine (SAS). We exposed A172 and T98G human glioblastoma cells to a presumably clinically relevant concentration of TMZ (25 µM) and/or 0.5 mM SAS for 1, 3, or 5 days and assessed cell viability. For both cell lines, TMZ alone did not alter viability at any time point, while the coadministration of TMZ and SAS significantly reduced cell viability after 5 days. The drug combination exerted a synergistic effect on A172 cells after 3 and 5 days. Therefore, this particular lineage was subjected to complementary analyses on the genetic (transcriptome) and functional (glutathione and proliferating cell nuclear antigen (PCNA) protein) levels. Cellular pathways containing differentially expressed genes related to the cell cycle were modified by TMZ alone. On the other hand, SAS regulated pathways associated with glutathione metabolism and synthesis, irrespective of TMZ. Moreover, SAS, but not TMZ, depleted the total glutathione level. Compared with the vehicle-treated cells, the level of PCNA protein was lower in cells treated with TMZ alone or in combination with SAS. In conclusion, our data showed that the association of TMZ and SAS is cytotoxic to T98G and A172 cells, thus providing useful insights for improving TMZ clinical efficacy through testing this novel drug combination. Moreover, the present study not only reports original information on differential gene expression in glioblastoma cells exposed to TMZ and/or SAS but also describes an antiproliferative effect of TMZ, which has not yet been observed in A172 cells.






Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) European organisation for research and treatment of cancer brain tumor and radiotherapy groups radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Yoshino A, Ogino A, Yachi K, Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E, Tsumoto K (2010) Gene expression profiling predicts response to temozolomide in malignant gliomas. Int J Oncol 36:1367–1377. doi:10.3892/ijo_00000621
Sun S, Wong TS, Zhang XQ, Pu JK, Lee NP, Day PJ, Ng GK, Lui WM, Leung GK (2012) Protein alterations associated with temozolomide resistance in subclones of human glioblastoma cell lines. J Neurooncol 107:89–100. doi:10.1007/s11060-011-0729-8
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165. doi:10.1038/nprot.2006.378
Sontheimer H (2008) A role for glutamate in growth and invasion of primary brain tumors. J Neurochem 105:287–295. doi:10.1111/j.1471-4159.2008.05301.x
Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H (2005) Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 25:7101–7110. doi:10.1523/JNEUROSCI.5258-04.2005
Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H (2007) Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res 67:9463–9471. doi:10.1158/0008-5472.CAN-07-2034
Robe PA, Bentires-Alj M, Bonif M, Rogister B, Deprez M, Haddada H, Khac MT, Jolois O, Erkmen K, Merville MP, Black PM, Bours V (2004) In vitro and in vivo activity of the nuclear factor-kappaB inhibitor sulfasalazine in human glioblastomas. Clin Cancer Res 10:5595–5603. doi:10.1158/1078-0432.CCR-03-0392
Chung WJ, Sontheimer H (2009) Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-kappaB. J Neurochem 110:182–193. doi:10.1111/j.1471-4159.2009.06129.x
Kanzawa T, Bedwell J, Kondo Y, Kondo S, Germano IM (2003) Inhibition of DNA repair for sensitizing resistant glioma cells to temozolomide. J Neurosurg 99:1047–1052. doi:10.3171/jns.2003.99.6.1047
Ostermann S, Csajka C, Buclin T, Leyvraz S, Lejeune F, Decosterd LA, Stupp R (2004) Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res 10:3728–3736. doi:10.1158/1078-0432.CCR-03-0807
Barazzuol L, Jena R, Burnet NG, Jeynes JC, Merchant MJ, Kirkby KJ, Kirkby NF (2012) In vitro evaluation of combined temozolomide and radiotherapy using X rays and high-linear energy transfer radiation for glioblastoma. Radiat Res 177:651–662. doi:10.1667/RR2803.1
Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69:581–593. doi:10.1046/j.1471-4159.1997.69020581.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354. doi:10.1073/pnas.76.9.4350
Vieira AS, Rezende AC, Grigoletto J, Rogério F, Velloso LA, Skaper SD, Negro A, Langone F (2009) Ciliary neurotrophic factor infused intracerebroventricularly shows reduced catabolic effects when linked to the TAT protein transduction domain. J Neurochem 110:1557–1566. doi:10.1111/j.1471-4159.2009.06259.x
Ignarro RS, Vieira AS, Sartori CR, Langone F, Rogério F, Parada CA (2013) JAK2 inhibition is neuroprotective and reduces astrogliosis after quinolinic acid striatal lesion in adult mice. J Chem Neuroanat 48–49:14–22. doi:10.1016/j.jchemneu.2013.02.005
Romero-Calvo I, Ocón B, Martínez-Moya P, Suárez MD, Zarzuelo A, Martínez-Augustin O, de Medina FS (2010) Reversible Ponceau staining as a loading control alternative to actin in western blots. Anal Biochem 401:318–320. doi:10.1016/j.ab.2010.02.036
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63. doi:10.1038/nrg2484
Huang H, Lin H, Zhang X, Li J (2012) Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol Rep 27:2050–2056. doi:10.3892/or.2012.1715
Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S (2004) Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ 11:448–457. doi:10.1038/sj.cdd.4401359
He W, Liu R, Yang SH, Yuan F (2015) Chemotherapeutic effect of tamoxifen on temozolomide-resistant gliomas. Anti Cancer Drugs 26:293–300. doi:10.1097/CAD.0000000000000197
Sleire L, Skeie BS, Netland IA, Førde HE, Dodoo E, Selheim F, Leiss L, Heggdal JI, Pedersen PH, Wang J, Enger PØ (2015) Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc−, leading to glutathione depletion. Oncogene 34:5951–5959. doi:10.1038/onc.2015.60
Mandal PK, Seiler A, Perisic T, Kölle P, Banjac Canak A, Förster H, Weiss N, Kremmer E, Lieberman MW, Bannai S, Kuhlencordt P, Sato H, Bornkamm GW, Conrad M (2010) System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J Biol Chem 285:22244–22253. doi:10.1074/jbc.M110.121327
Musacchio A, Hardwick KG (2002) The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol 3:731–741. doi:10.1038/nrm929
Morales AG, Pezuk JA, Brassesco MS, de Oliveira JC, de Paula Queiroz RG, Machado HR, Carlotti CG, Neder L, de Oliveira HF, Scrideli CA, Tone LG (2013) BUB1 and BUBR1 inhibition decreases proliferation and colony formation, and enhances radiation sensitivity in pediatric glioblastoma cells. Childs Nerv Syst 29:2241–2248. doi:10.1007/s00381-013-2175-8
Wang L, Zhang J, Wan L, Zhou X, Wang Z, Wei W (2015) Targeting Cdc20 as a novel cancer therapeutic strategy. Pharmacol Ther 151:141–151. doi:10.1016/j.pharmthera.2015.04.002
Marucci G, Morandi L, Magrini E, Farnedi A, Franceschi E, Miglio R, Calò D, Pession A, Foschini MP, Eusebi V (2008) Gene expression profiling in glioblastoma and immunohistochemical evaluation of IGFBP-2 and CDC20. Virchows Arch 453:599–609. doi:10.1007/s00428-008-0685-7
Zeng X, Sigoillot F, Gaur S, Choi S, Pfaff KL, Oh DC, Hathaway N, Dimova N, Cuny GD, King RW (2010) Pharmacologic inhibition of the anaphase-promoting complex induces a spindle checkpoint-dependent mitotic arrest in the absence of spindle damage. Cancer Cell 18:382–395. doi:10.1016/j.ccr.2010.08.010
Ivanchuk SM, Rutka JT, Piepmeier JM, Parsa AT, Boulis N (2004) The cell cycle: accelerators, brakes, and checkpoints. Neurosurgery 54:692–700. doi:10.1227/01.NEU.0000109534.28063.5D
Mukherjee M, Byrd T, Brawley VS, Bielamowicz K, Li XN, Merchant F, Maitra S, Sumazin P, Fuller G, Kew Y, Sun D, Powell SZ, Ahmed N, Zhang N, Pati D (2014) Overexpression and constitutive nuclear localization of cohesin protease separase protein correlates with high incidence of relapse and reduced overall survival in glioblastoma multiforme. J Neurooncol 119:27–35. doi:10.1007/s11060-014-1458-6
Hirose Y, Berger MS, Pieper RO (2001) p53 effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res 61:1957–1963
Shen W, Hu J-A, Zheng J-S (2014) Mechanism of temozolomide-induced antitumour effects on glioma cells. J Int Med Res 42:164–172. doi:10.1177/0300060513501753
Jo MY, Kim YG, Kim Y, Lee SJ, Kim MH, Joo KM, Kim HH, Nam DH (2012) Combined therapy of temozolomide and ZD6474 (vandetanib) effectively reduces glioblastoma tumor volume through anti-angiogenic and anti-proliferative mechanisms. Mol Med Rep 6:88–92. doi:10.3892/mmr.2012.868
Son MJ, Kim JS, Kim MH, Song HS, Kim JT, Kim H, Shin T, Jeon HJ, Lee DS, Park SY, Kim YJ, Kim JH, Nam DH (2006) Combination treatment with temozolomide and thalidomide inhibits tumor growth and angiogenesis in an orthotopic glioma model. Int J Oncol 28:53–59. doi:10.3892/ijo.28.1.53
Backos DS, Franklin CC, Reigan P (2012) The role of glutathione in brain tumor drug resistance. Biochem Pharmacol 83:1005–1012. doi:10.1016/j.bcp.2011.11.016
Aquilano K, Baldelli S, Ciriolo MR (2014) Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol 5:196. doi:10.3389/fphar.2014.00196
Balendiran GK, Dabur R, Fraser D (2004) The role of glutathione in cancer. Cell Biochem Funct 22:343–352. doi:10.1002/cbf.1149
Traverso N, Ricciarelli R, Nitti M, Marengo B, Furfaro AL, Pronzato MA, Marinari UM, Domenicotti C (2013) Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev 2013:972913
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:972913. doi:10.1146/annurev.pharmtox.45.120403.095857
Robe PA, Martin DH, Nguyen-Khac MT, Artesi M, Deprez M, Albert A, Vanbelle S, Califice S, Bredel M, Bours V (2009) Early termination of ISRCTN45828668, a phase 1/2 prospective, randomized study of sulfasalazine for the treatment of progressing malignant gliomas in adults. BMC Cancer 9:372. doi:10.1186/1471-2407-9-372
Takeuchi S, Wada K, Nagatani K, Otani N, Osada H, Nawashiro H (2014) Sulfasalazine and temozolomide with radiation therapy for newly diagnosed glioblastoma. Neurol India 62:42–47. doi:10.4103/0028-3886.128280
Chen L, Li X, Liu L, Yu B, Xue Y, Liu Y (2015) Erastin sensitizes glioblastoma cells to temozolomide by restraining xCT and cystathionine-γ-lyase function. Oncol Rep 33:1465–1474. doi:10.3892/or.2015.3712
Funding
This work was supported by Grants from FAPESP (2011/50400-0; 2013/02618-1; 2013/07559-3) and FAEPEX/UNICAMP (379/13; 554/14; 621/14). RSI was a recipient of a scholarship from CAPES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We have read and have abided by the statement of ethical standards for manuscripts submitted to Molecular and Cellular Biochemistry. This paper is not concurrently under consideration for publication in any other journal.
Conflicts of interest
The authors declare that all authors are in agreement with the present submission and have no conflicts of interest.
Research involving human and animal participants
In addition, this research does not encompass any studies involving human participants or animals performed by any of the authors.
Ethical approval
This research does not encompass any studies involving human participants or animals performed by any of the authors.
Additional information
Raffaela Silvestre Ignarro and Gustavo Facchini contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2016_2742_MOESM1_ESM.xls
Online Resource 1 Gene expression data in A172 human glioblastoma cells after 3 days of treatment with temozolomide (TMZ) and/or sulfasalazine (SAS). Columns in Tables 1–3 show the gene symbol, log2 fold change relative to 0.1 % DMSO-treated cells, p value and adjusted p value. Tables 4–7 contain the enriched pathways for the up- and downregulated genes for each group. Fold change for each gene in the enriched pathways is presented in parenthesis after the name of the gene (XLS 6870 kb)
Rights and permissions
About this article
Cite this article
Ignarro, R.S., Facchini, G., Vieira, A.S. et al. Sulfasalazine intensifies temozolomide cytotoxicity in human glioblastoma cells. Mol Cell Biochem 418, 167–178 (2016). https://doi.org/10.1007/s11010-016-2742-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-016-2742-x