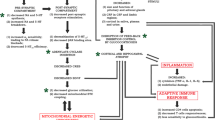
This article discusses studies of the pathophysiological bases of the depressive state reflecting the significance of the third component of signal transmission in the pathogenesis of depression. Published data are summarized and the characteristics of tryptophan metabolism, impairments to serotonin and noradrenaline metabolism, and dysfunction of serotoninergic, noradrenergic, and dopaminergic neurotransmission in depression are discussed. The role of neurotrophins as first messengers regulating cellular functional activity is analyzed. Impairments to neurotrophin functions lead to a shift between the processes of proliferation and apoptosis towards the latter, which causes a reduction in neurogenesis and the development of neurodegeneration in pathological states, particularly depressive disorder.
Similar content being viewed by others
References
A. A. Borodinova, A. B. Zyuzina, and P. M. Balaban, “The role of atypical protein kinases in maintaining long-term memory and synaptic plasticity,” Biokhimiya, No. 2, 372 (2017).
O. S. Brusov, M. I. Faktor, and F. B. Katasonov, “Structural and functional changes in the brain in emotional disorders: the bases of the neurocirculatory and neurotrophic hypotheses of depression,” Zh. Nevrol. Psikhiat., 7, 83 (2012).
O. A. Gomazkov, Neurogenesis as an Adaptive Function of the Brain, Ikar, Moscow (2013).
O. A. Gomazkov, “Neurogenesis as an organizing function of the adult brain. Is there sufficient evidence,” Usp. Sovr. Biol., 136, No. 3, 227 (2016).
O. A. Gomazkov, “Signal molecules as regulators of neurogenesis in the adult brain,” Neirokhimiya, 30, 1 (2013).
G. A. Grigor’yan, N. N. Dygalo, A. B. Gekht, et al., “Molecular and cellular mechanisms of depression. The role of glucocorticoids, cytokines, neurotransmitters, and trophic factors in the genesis of depressive disorders,” Usp. Fiziol. Nauk., 45, No. 2, 3 (2014).
E. E. Dubinina, Metabolic Products of Oxygen in the Functional Activity of Cells (life and death, creation and destruction), Medical Press, Moscow (2006).
E. E. Dubinina, L. V. Shchedrina, and G. E. Mazo, “Main biochemical aspects of the pathogenesis of depression. Part I,” Usp. Fiziol. Nauk., No. 1, 28 (2018).
E. E. Dubinina, L. V. Shchedrina, G. E. Mazo, et al., “Neurogenesis and neurodegeneration processes in depressive disorders,” Psikhich. Zdorov., 122, No. 7, 29 (2016).
E. E. Dubinina, L. V. Shchedrina, N. G. Neznanov, et al., “Oxidative stress and its influence on the functional activity of cells in Alzheimer’s disease,” Biomed. Khim., No. 1, 57 (2015).
E. V. Dubynina and O. V. Dolotov, “Transcription factor CREB and memory formation processes,” Neirokhimiya, 26, No. 3, 181 (2009).
N. D. Eshchenko, Biochemistry of Mental and Nervous Diseases, St. Petersburg State University, St. Petersburg (2004).
T. V. Zhilyaeva and L. N. Kasimova, “Impairments to one-carbon metabolism,” in: Depression and the Risk of Developing Somatic Diseases, N. G. Neznanov et al. (eds.), Specialist Medical Books Publisher, Moscow (2018).
T. V. Zhilyaeva, V. I. Larionova, and G. E. Mazo, “Pterins as potential substances for overcoming therapeutic resistance in schizophrenia,” Sovremen. Ter. Psikhich. Rasstr., No. 1, 2 (2018).
G. Kaplan and B. Sédok, Clinical Psychiatry, Geotar Medicine, Moscow (1998).
E. D. Kas’yanov and G. E. Mazo, “A clinical case of hyperhomocysteinemia and recurrent depressive disorder,” Farmakogenet. Farmakogenom., No. 2, 53 (2018).
E. D. Kas’yanov and G. E. Mazo, “Functioning of the hypothalamo-hypophyseal-adrenal axis in depression: current state of the problem,” Psikhich. Zdorov., No. 8, 27 (2017).
N. K. Popova, T. V. Il’chibaeva, and V. S. Naumenko, “Neurotrophic factors (BDNF, GDNF) and the serotoninergic system of the brain,” Biokhimiya, 82, No. 3, 449 (2017).
A. S. Tiganov, G. I. Kopeiko, O. S. Brusov, and T. P. Klyushnik, “Progress in studies of the pathogenesis and treatment of endogenous depression,” Zh. Nevrol. Psikhiat., 11, 65 (2012).
M. G. Uzbekov, and E. Yu. Misionzhnik, “Nonspecific endogenous intoxication syndrome as an integral component of the pathogenesis of mental disorders,” Ross. Psikhiat. Zh., 4, 56 (2000).
J. R. Albani, “Motions of tryptophan residues in asialylated human alpha 1-acid glycoprotein,” Biochim. Biophys. Acta, 1291, No. 3, 215 (1996).
S. J. Allen, J. J. Watson, D. K. Shoemark, et al., “GDNF, NGF and BDNF as therapeutic options for neurodegeneration,” Pharmacol. Ther., 138, No. 2, 155 (2013).
F. Angelucci, S. Brene, and A. A. Mathe, “BDNF in schizophrenia depression and corresponding animal models,” Mol. Psychiatry, 10, No. 4, 345 (2005).
F. Artigas, “Serotonin receptors involved in antidepressant effects,” Pharmacol. Ther., 137, 119 (2013).
A. E. Autry and L. M. Monteggia, “Brain-derived neurotrophic factor and neuropsychiatric disorders,” Pharmacol. Res., 64, 238 (2012).
H. Baran, K. Staniek, B. Kepplinger, et al., “Kynurenines and the respiratory parameters on rat heart mitochondria,” Life Sci., 72, No. 10, 1103 (2003).
N. M. Barnes and T. A. Sharp, “A review of central 5-HT receptors and their junction,” Neuropsychopharmacology, 38, 1083 (1999).
G. L. Barrett, “The p75 neurotrophin receptor and neuronal apoptosis,” Prog. Neurobiol., 61, 205 (2000).
R. Barrientos, D. V. Sprunger, S. Campeau, et al., “Brain-derived neurotrophic factor mRNA downregulation induced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist,” Neuroscience, 121, 847 (2003).
M. J. Berridge, Cell Signaling Biology. Module 1. Introduction (2012), https://doi.org/https://doi.org/10.1042/csb0001001.
M. J. Berridge, Cell Signaling Biology. Module 2. Cell Signaling Pathway (2012), https://doi.org/https://doi.org/10.1042/csb0001002.
M. J. Berridge, Cell Signaling Biology. Module 4. Sensors and Effectors, https://doi.org/https://doi.org/10.1042/csb00010 (2012).
M. J. Berridge, Cell Signaling Biology. Module 9. Cell Cycle and Proliferation (2012), https://doi.org/https://doi.org/10.1042/csb0001009
E. Beurel, S. F. Grieco, and R. S. Jope, “Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases,” Pharmacol. Ther., 148, 114 (2015).
J. M. Brezun and A. Daszuta, “Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats,” Neuroscience, 89, 999 (1999).
A. Brunet, A. Bonni, M. J. Zigmond, et al., “Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor,” Cell, 96, No. 6, 857 (1999).
C. G. Causing, A. Gloster, R. Aloyz, et al., “Synaptic innervation density is regulated by neuron-derived BDNF,” Neuron, 18, No. 2, 257 (1997).
M. V. Chao and M. Botwell, “Neurotrophins: to cleave or not to cleave,” Neuron, 33, 9 (2002).
C. T. Chu, D. J. Levinthal, S. M. Kulich, et al., “Oxidative neuronal injury. The dark side of ERK1/2,” Eur. J. Biochem., 271, No. 11, 2060 (2004).
A. Cole, S. Frame, and P. Cohen, “Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event,” Biochem. J., 377, No. 1, 249 (2004).
T. G. Connor, N. O. Star, J. B. Sullivan, and A. Harkin, “Introduction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma,” Neurosci. Lett., 441, No. 1, 29 (2008).
A. Currais, T. Hortobagyi, and S. Soriano, “The neuronal cell cycle as a mechanism of pathogenesis in Alzheimer’s disease,” Aging (Albany NY), 1, No. 4, 363 (2009).
M. P. Czech, “PIP2 and PIP3 complex roles at the cell surface,” Cell, 100, 603 (2000).
R. Dantzer, J. C. O’Connor, M. A. Lawson, and K. W. Kelley, “Inflammation- associated depression: From serotonin to kynurenine,” Psychoneuroendocrinology, 36, 426 (2011).
S. R. Datta, “Akt phosphorylation of BAD couples survival to cell-machinery,” Cell, 91, 231 (1997).
L. Del Peso, M. Gonzalez-Garcia, C. Page, et al., “Interleukin-3- induced phosphorylation of BAD through the protein kinase,” Science, 278, 687 (1997).
G. Di Paolo and P. De Camilli, “Phosphoinositides in cell regulation and membrane dynamics. Review Article,” Nature, 443, 651 (2006).
R. S. Duman, “Role of neurotrophic factors in the etiology and treatment of mood disorders,” Neuromolecular Med., 5, 11 (2004).
R. S. Duman and L. M. Monteggia, “A neurotrophic model for stress related mood disorders,” Biol. Psychiatry, 59, 1116 (2006).
S. Dunham, J. F. Deakin, F. Miyajima, et al., “Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains,” J. Psychiatr. Res., 43, 1175 (2009).
F. Esposito, G. Chirico, N. Montesano Gesualdi, et al., “Protein kinase B activation by reactive oxygen species is independent of tyrosine kinase receptor phosphorylation and requires Src activity,” J. Biol. Chem., 278, No. 23, 20,828 (2003).
M. Fava and D. Mischoulon, “Folate in depression: efficacy, safety, differences in formulations, and clinical issues,” J. Clin. Psychiatry, 70, No. 5, 12 (2009).
B. S. Fernandes, C. S. Gama, K. M. Cereser, et al., “Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis,” J. Psychiatr. Res., 45, 995 (2011).
A. J. M. Filho, C. N. C. Lima, S. N. N. Vasconcelos, et al., “IDO chronic immune activation and tryptophan metabolic pathway: A potential pathophysiological link between depression and obesity,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 80, 234 (2018).
V. Gabbay, R. G. Klein, Y. Katz, et al., “The possible role of the kynurenine pathway in adolescent depression with melancholic features,” J. Child Psychol. Psychiatry, 51, No. 8, 935 (2010).
R. H. Goodman and S. Smolik, “CBP/p300 in cell growth, transformation, and development,” Genes Dev., 14, No. 13, 1553 (2000).
R. G. Graeff, P. S. Guimaraes, T. G. De Andrade, and J. F. Deakin, “Role of SHT in stress, anxiety and depression,” Pharm. Biochem. Behav., 54, 129 (1998).
R. S. Grant, Y. Naif, M. Espinola, et al., “IDO after INF-gamma activated astroglia: a role in improving cell viability during oxidative stress,” Redox Rep., 5, 101 (2000).
G. J. Guillemin, S. J. Kerr, G. A. Smythe, et al., “Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection,” J. Neurochem., 78, No. 4, 842 (2001).
G. J. Guillemin, G. Smythe, O. Tokikawa, et al., “Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons,” Glia, 49, 15 (2005).
K. Z. Guyton, Y. Liu, M. Gorospe, et al., “Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury,” J. Biol. Chem., 271, No. 8, 4138 (1996).
J. Haase and E. Brown, “Integrating the monoamine, neurotrophin and cytokine hypothesis of depression – A central role for the serotonin transporter,” Pharmacol. Ther., 147, 1 (2015).
P. T. Hawkins, K. E. Anderson, K. Davidson, and L. R. Stephens, “Signalling through class I PI3Ks in mammalian cells,” Biochem. Soc. Trans., 34, 647 (2006).
C. H. Heldin, “Protein tyrosine kinase receptor signaling overview,” R. A. Bradshaw and E. A. Dennis (eds.), Academic Press, San Diego (2003).
F. Henn, B. Vollmayer, and A. Sartorius, “Mechanism of depression: the role of neurogenesis,” Drug Discov. Today Dis. Mech., 1, 407 (2004).
S. Herold, R. Jagasia, K. Merz, et al., “CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis,” Mol. Cell. Neurosci., 46, No. 1, 79 (2011).
I. Hindmarch, “Beyond the monoamine hypothesis: mechanisms, molecules and methods,” Eur. Psychiatry, 17, No. 3, 294 (2002).
E. J. Huang and L. F. Peichardt, “Neurotrophins: roles in neuronal development and function,” Annu. Rev. Neurosci., 24, 677 (2001).
A. Ianari, R. Gallo, M. Palma, et al., “Specific role for p300/CREBbinding protein-associated factor activity in E2F1 stabilization in response to DNA damage,” J. Biol. Chem., 279, No. 29, 30830 (2004).
F. Karege, G. Vaudan, M. Schwald, et al., “Neurotrophin levels in postmortem brains of suicide and the effects of antemortem diagnosis and psychotropic drugs,” Brain Res. Mol. Brain Res., 136, 29 (2005).
K. Karg, M. Burmeister, K. Shedden, et al., “The serotonin transporter promoter variant (5-HTTLPR), stress and depression meta-analysis revisited: Evidence of genetic moderation,” Arch. Gen. Psychiatry, 68, 444 (2011).
K. Kobayashi, K. Hayashi, and M. Sono, “Effects of tryptophan and pH on the kinetics of superoxide radical binding to indoleamine 2,3-dioxygenase studied by pulse radiolysis,” J. Biol. Chem., 264, No. 26, 15280 (1989).
R. Kobrosly and E. van Wijngaarden, “Associations between immunologic, inflammatory, and oxidative stress markers with severity of depressive symptoms: An analysis of the 2005–2006 National Health and Nutrition Examination Survey,” Neurotoxicology, 31, 126 (2010).
M. Korte, P. Carroll, E. Wolf, et al., “Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor,” Proc. Natl. Acad. Sci. USA, 92, 8856 (1995).
V. Krishnan and E. J. Nestler, “The molecular neurobiology of depression,” Nature, 455, 894 (2008).
M. Kubera, E. Obuchowicz, L. Goehler, et al., “In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 35, 744 (2011).
A. V. Kulikov, V. S. Naumenko, A. S. Tsybko, et al., “The role of glycoprotein gp 130 in serotonin mediator system in mouse brain,” Mol. Biol. (Mosk.), 44, No. 4, 801 (2010).
R. Kuruvilla, H. Ye, and D. D. Ginty, “Spatially and functionally district roles of the PI3-K effector pathway during NGF signaling in sympathetic neurons,” Neuron, 27, 499 (2000).
A. Laugeray, J. M. Launay, J. Callebert, et al., “Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression,” Behav. Brain Res., 210, No. 1, 84 (2010).
F. S. Lee, A. N. Kim, G. Khursigara, et al., “The uniqueness of being a neurotrophin receptor,” Curr. Opin. Neurobiol., 11, 281 (2001).
B. Leonard and M. Maes, “Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression,” Neurosci. Biobehav. Rev., 36, 764 (2012).
D. C. Lie, S. A. Colamarino, H. J. Song, et al., “Wnt signaling regulates adult hippocampal neurogenesis,” Nature, 437, No. 7063, 1370 (2005).
J. M. Loftis, M. Huckans, and B. J. Morasco, “Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies,” Neurobiol. Dis., 37, 519 (2010).
C. Y. Logan and R. Nusse, “The Wnt signaling pathway in development and disease,” Ann. Rev. Cell Dev. Biol., 20, 781 (2004).
B. Lu, “BDNF and activity-dependent synaptic modulation,” Learn. Mem., 10, No. 2, 86 (2003).
B. Lu, P. T. Pang, and N. H. Woo, “The yin and yang of neurotrophin action,” Neurosci., 6, 603 (2005).
G. M. Mackay, C. M. Forrest, J. Christofides, et al., “Kynurenine metabolites and inflammation markers in depressed patients treated with fluoxetine or counselling,” Clin. Exp. Pharmacol. Physiol., 36, No. 4, 425 (2009).
M. Maes, “Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 35, No. 3, 664 (2011).
M. Maes, “Evidence for an immune response in major depression: a review and hypothesis,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 19, 11 (1995).
M. Maes, “Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression,” Neurosci. Biobehav. Rev., 36, 764 (2012).
M. Maes, “The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress and leaky gut as new targets for adjunctive treatments in depression,” Neuroendocrinol. Lett., 29, No. 3, 287 (2008).
M. Maes, P. Galecki, Y. S. Chang, and M. Berk, “A review on the oxidative and nitrosative stress [O&NS] pathways in major depression and their possible contribution to the neurodegenerative processes in that illness,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 35, No. 3, 676 (2011).
M. Maes, P. Galecki, R. Verkerk, and W. Rief, “Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity,” Neuroendocrinol. Lett., 32, No. 3, 264 (2011).
M. Maes, B. F. Leonard, A. M. Myint, et al., “The new 5-HT hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 35, No. 3, 702 (2011).
M. Maes and H. V. Meltzer, “The serotonin hypothesis of major depression. Selected chapters on mood disorders,” in: The Fourth Generation of Progress, F. Bloom and D. Kupfer (ed.), Raven Press, USA (1995).
M. Maes, L. Michaylova, M. Kubera, et al., “Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in (ME/CFS) due to cardiovascular disorder, Neuroendocrinol. Lett., 30, No. 4, 470 (2009).
M. Maes, L. Michaylova, and J. C. Leunis, “Increased serum IgM antibodies directed against phosphatidyl inositol in chronic fatigue syndrome (CFS) and major depression: evidence that an IgMmediated immune response against Pi is one factor underpinning the comorbidity between both CFS and depression,” Neuroendocrinol. Lett., 28, No. 6, 861 (2007).
M. Maes, I. Mihaylova, M. D. Ruyter, et al., “The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression – and other conditions characterized by tryptophan depletion induced by inflammation,” Neuroendocrinol. Lett., 28, No. 6, 826 (2007).
M. Maes, R. Verkerk, S. Bonaccorso, et al., “Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation,” Life Sci., 71, No. 16, 1837 (2002).
M. Maes, R. Yirmyia, J. Noraberg, et al., “The inflammatory and neurodegenerative [I&ND] hypothesis of depression: leads for future research and new drug developments in depression,” Metab. Brain Dis., 24, 27 (2009).
M. Majdan and F. Miller, “Neuronal life and death decisions: functional antagonism between the Trk and p75 neurotrophin receptors,” Int. J. Dev. Neurosci., 17, 153 (1999).
G. S. Malhi, G. B. Parker, and J. Greenwood, “Structural and functional models of depression: from sub-types to substrates,” Acta Psychiatr. Scand., 111, 94 (2005).
S. Malynn, A. Campos-Torres, P. Moynagh, and J. Haase, “The pro-inflammatory cytokine TNF-alfa regulates the activity and expression of the serotonin transporter (SERT) in astrocytes,” Neurochem. Res., 38, 694 (2013).
L. A. Mamounas, M. E. Blue, J. A. Siuciak, and C. A. Altar, “Brainderived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain,” J. Neurosci., 15, 7929 (1995).
M. P. Mattson, S. Maudsley, and B. Martin, “BDNF and 5HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders,” Trends Neurosci., 10, 589 (2004).
T. B. Meier, W. C. Drevets, B. E. Wurfel, et al., “Relationship between neurotoxic kynurenine metabolites and reductions in right medial prefrontal cortical thickness in major depressive disorder,” Brain Behav. Immun., 53, 39 (2016).
A. L. Miller, “The methylation, neurotransmitter, and antioxidant connections between folate and depression,” Altern. Med. Rev, 13, No. 3, 216 (2008).
A. A. Moustafa, H. H. Doaa, and M. E. Abeer, et al., “Homocysteine levels in schizophrenia and affective disorders focus on cognition,” Front. Behav. Neurosci., 8, 343 (2014).
N. Muller and M. J. Schwarz, “COX-2 inhibition in schizophrenia and major depression,” Curr. Pharm. Des., 14, 1452 (2008).
N. Muller and M. J. Schwarz, “The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression,” Mol. Psychiatry, 12, 988 (2007).
D. L. Murphy, M. A. Fox, K. R. Timpano, et al., “How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems,” Neuropharmacology, 55, 932 (2008).
A. M. Myint, Y. K. Kim, R. Verkerk, et al., “Kynurenine pathway in major depression: evidence of impaired neuroprotection,” J. Affect. Disord., 98, No. 1–2, 143 (2007).
V. S. Naumenko, E. M. Kondaurova, and N. K. Popova, “Effect of brain-derived neurotrophic factor on behavior and key members of the brain serotonin system in genetically predisposed to behavioral disorders mouse strains,” Neuroscience, 214, 59 (2012).
W. J. Nelson and R. Nusse, “Convergence of Wnt, beta-catenin, and cadherin pathways,” Science, 303, No. 5663, 1483 (2004).
A. Nykjser, R. Lee, K. Teng, et al., “Sortilin is essential for proNGF-induced neuronal cell death,” Nature, 427, 843 (2004).
O. Ogawa, H. G. Lee, X. Zhu, et al., “Increased p27, an essential component of cell cycle control, in Alzheimer’s disease,” Aging Cell, 2, No. 2, 105 (2003).
T. Okajima, Y. Kawata, and K. Hamaguchi, “Chemical modification of tryptophan residues and stability changes in proteins,” Biochemistry, 29, No. 39, 9168 (1990).
K. O. Ozlem, K. C. Doberauer, K. C.Vadodaria, et al., “Prosperorelated homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has stage-specific role in adult hippocampal neurogenesis,” Proc. Natl. Acad. Sci. USA, 108, No. 14, 5807 (2011).
A. A. Piccinni, “Del Debbio, and P. Medda, et al., “Plasma Brain- Derived Neurotrophic Factor in treatment-resistant depressed patients receiving electroconvulsive therapy,” Eur. Neuropsychopharmacol., 19, 349 (2009).
H. Plein and M. Berk, “Changes in the platelet intracellular calcium response to serotonin in patients with major depression treated with electroconvulsive therapy: state or trait marker status,” Int. Clin. Psychopharmacol., 15, No. 2, 93 (2000).
H. Plein, M. Berk, S. Eppel, and N. Butkow, “Augmented platelet calcium uptake in response to serotonin stimulation in patients with major depression measured using Mn2+ influx and 45Ca2+ uptake,” Life Sci., 66, No. 5, 423 (2000).
A. M. Polter, S. Yang, and R. S. Jope, “Functional significance of glycogen synthase kinase-3 regulation by serotonin,” Cell. Signal., 24, 265 (2012).
L. Ruan, B. Wui-Man Lau, and J. Wang, et al., “Neurogenesis in neurological and psychiatric diseases and brain injury: From bench to bedside,” Prog. Neurobiol., 115, 116 (2014).
P. Rumajogee, A. Madeira, D. Verge, et al., “Up-regulation of the neuronal serotoninergic phenotype in vitro: BDNF and cAMP share Trk B-dependent mechanisms,” J. Neurochem., 83, 1525 (2002).
M. T. Sapko, P. Guidetti, P. Yu, et al., “Endogenous kynurenate controls the vulnerability of striatal neurons to quinolinate: implications for Huntington’s disease,” Exp. Neurol., 197, No. 1, 31 (2006).
J. H. Schafer, T. A. Glass, K. I. Bolla, et al., “Homocysteine and cognitive function in a population–based study of older adults,” J. Am. Geriatr. Soc., 53, 381 (2005).
L. Schiller, M. Donix, M. Jähkel, and J. Oehler, “Serotonin 1A and 2A receptor densities, neurochemical and behavioural characteristics in two closely related mice strains after long-term isolation,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 30, 492 (2006).
P. F. Schuck, A. Tonin, G. da Costa Ferreira, et al., “Kynurenines impair energy metabolism in rat cerebral cortex,” Cell. Mol. Neurobiol., 27, No. 1, 147 (2007).
S. Sen, R. Duman, and G. Sanacora, “Serum brain-derived neurotrophic factor, depression, and antidepressant medications: Metaanalyses and implications,” Biol. Psychiatry, 64, 527 (2008).
P. J. Shah, M. F. Glabus, G. M. Goodwin, and K. P. Ebmeier, “Chronic, treatment-resistant depression and right fronto-striatal atrophy,” Br. J. Psychiatry, 180, 434 (2002).
M. V. Sofroniew, C. L. Howe, and W. C. Mobley, “Nerve growth factor signaling, neuroprotection, and neural repair,” Annu. Rev. Neurosci., 24, 1217 (2001).
D. Stauffer, B. Chang, and J. Huang, et al., “p300/CREB-binding protein interacts with ATR and is required for the DNA replication checkpoint,” J. Biol. Chem., 282, No. 13, 9678 (2007).
K. J. Swartz, M. J. During, A. Freese, and M. F. Beal, “Cerebral synthesis and release of kynurenic acid: an endogenous antagonist of excitatory amino acid receptors,” J. Neurosci., 10, No. 9, 2965 (1990).
M. Talowska, A. Orzechowska, J. Szemraj, et al., “Manganese superoxide dismutase gene expression and cognitive functions in recurrent depressive disorder,” Neuropsychology, 70, No. 1, 23 (2014).
L. Tapia-Aruncibia, F. Rage, L. Givalous, and S. Aruncibia, “Physiology of BDNF: focus on hypothalamic function,” Front. Neuroendocrinol., 25, 77 (2004).
R. G. Tavares, C. I. Tasca, and C. E. Santos, et al., “Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes,” Neurochem. Int., 40, 621 (2002).
A. Terracciano, M. Lobina, M. G. Piras, et al., “Neuroticism, depressive symptoms, and serum BDNF,” Psychosom. Med., 73, No. 8, 638 (2011).
G. E. Torres, R. R. Gainetdinov, and M. G. Caron, “Plasma membrane monoamine transporters: Structure, regulation and function,” Nat. Rev. Neurosci., 4, 13 (2003).
E. Undine, L. Lang, K. Günther, et al., “Higher BDNF concentrations in the hippocampus and cortex of an aggressive mouse strain,” Behav. Brain Res., 197, 246 (2009).
I. Vincent, C. I. Pae, and J. L. Hallows, “The cell cycle and human neurodegenerative disease,” Prog. Cell Cycle Res., 5, 31 (2003).
F. L. Watson, “Neurotrophins use the Erk5 pathway to mediate a retrograde survival response,” Nature Neurosci., 4, 981 (2001).
M. C. Wichers and M. Maes, “The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression,” J. Psychiatry Neurosci., 29, No. 1, 11 (2004).
Xingbing Huang, Xiong Huang, et al., “Association of serum BDNF levels with psychotic symptom in chronic patients with treatment-resistant depression in a Chinese Han population,” Psychiatry Res., 257, 279 (2017).
C. B. Zhu, R. D. Blakely, and W. A. Hewlett, “The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters,” Neuropsychopharmacology, 31, No. 10, 2121 (2006).
C. B. Zhu, K. M. Lindler, A. W. Owens, et al., “Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters,” Neuropsychopharmacology, 35, 2510 (2010).
L. S. Zweifel, R. Kuruvilla, and D. D. Ginty, “Functions and mechanisms of retrograde neurotrophin signaling,” Neurosci., 6, 615 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Uspekhi Fiziologicheskikh Nauk, Vol. 52, No. 1, pp. 31–48, January–March, 2021.
Rights and permissions
About this article
Cite this article
Dubinina, E.E., Schedrina, L.V. & Mazo, G.E. Main Biochemical Aspects of the Pathogenesis of Depression. Part II. Neurosci Behav Physi 51, 1330–1343 (2021). https://doi.org/10.1007/s11055-021-01198-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-021-01198-9