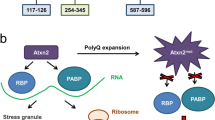
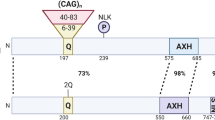
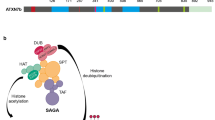
Type 2 spinocerebellar ataxia (SCA2) is an inherited progressive disease whose cause at the genetic level is an expansion of the polyglutamine tract in ataxin-2 protein. Effective treatment and disease-modifying therapy remain unavailable to patients with SCA2. Patients are currently given only symptomatic treatment, along with palliative medical care. With the aim of seeking new therapeutic targets for treatment of SCA2, many scientific groups have tried to study the physiological, molecular, and biochemical changes to cerebellar neurons in patients with SCA2 and in various model systems. State-of-the-art approaches to studies of the pathogenesis of SCA2 have yielded new data on the molecular mechanisms of the disease and have suggested possible strategies for the potential treatment of this disease. The present review summarizes current data on the genetic basis of SCA2, describes the known properties and functions of ataxin-2 protein, considers the mechanisms of degeneration of cerebellar cortex cells, impairments to their physiological function, and associated damage to the conducting pathways of the cerebellum, and presents data on contemporary model systems used for studies of the basis of SCA2; we also present information on novel approaches to studies of the molecular mechanisms underlying the pathology of SCA2 such as aggregation, oxidative stress, and damage to cell signaling and calcium signaling, and consider the role of autophagy and the microglia in the molecular pathogenesis of SCA2.
Similar content being viewed by others
References
T. Ashizawa, G. Oz, and H. L. Paulson, “Spinocerebellar ataxias: Prospects and challenges for therapy development,” Nat. Dev. Neurol., 14, No. 10, 590–605 (2018).
J. J. Magana, L. Velazquez-Perez, and B. Cisneros, “Spinocerebellar ataxia type 2: Clinical presentation, molecular mechanisms, and therapeutic perspectives,” Mol. Neurobiol., 47, No. 1, 90–104 (2013).
H. L. Paulson, V. G. Shakkottai, H. B. Clark, and H. T. Orr, “Polyglutamine spinocerebellar ataxias – from genes to potential treatments,” Nat. Rev. Neurosci., 18, No. 10, 613–626 (2017).
D. R. Scoles and S. M. Pulst, “Spinocerebellar ataxia type 2,” Adv. Exp. Med. Biol., 1049, 175–195 (2018).
R. A. M. Buijsen, L. J. A. Toonen, S. L. Gardiner, and W. M. C. van Roon-Mom, “Genetics, mechanisms, and therapeutic progress in polyglutamine spinocerebellar ataxias,” Neurotherapeutics, (2019).
T. F. Satterfield and L. J. Pallanck, “Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes,” Hum. Mol. Genet., 15, No. 16, 2523–2532 (2006).
J. M. Alves-Cruzeiro, L. Mendonca, L. Pereira de Almeida, and C. Nobrega, “Motor dysfunctions and neuropathology in mouse models of spinocerebellar ataxia type 2: A comprehensive review,” Front. Neurosci., 10, 572 (2016).
C. J. Smeets and D. S. Verbeek, “Climbing fi bers in spinocerebellar ataxia: A mechanism for the loss of motor control,” Neurobiol. Dis., 88, 96–106 (2016).
T. Takeuchi and Y. Nagai, “Protein misfolding and aggregation as a therapeutic target for polyglutamine diseases,” Brain Sci., 7, No. 10 (2017).
T. H. Massey and L. Jones, “The central role of DNA damage and repair in CAG repeat diseases,” Dis. Model Mech., 11, No. 1 (2018).
P. Egorova, E. Popugaeva, and I. Bezprozvanny, “Disturbed calcium signaling in spinocerebellar ataxias and Alzheimer’s disease,” Semin. Cell. Dev. Biol., 40, 127–133 (2015).
P. A. Egorova and I. B. Bezprozvanny, “Inositol-1,4,5-trisphosphate receptors and neurodegenerative disorders,” FEBS J., 285, No. 19, 3547–3565 (2018).
C. Hisatsune, K. Hamada, and K. Mikoshiba, “Ca(2+) signaling and spinocerebellar ataxia,” Biochem. Biophys. Acta Mol. Cell. Res., 11, 1733–1744 (2018).
M. D. Mark, J. C. Schwitalla, M. Groemmke, and S. Herlitze, “Keeping our calcium in balance to maintain our balance,” Biochem. Biophys. Res. Commun, 483, No. 4, 1040–1050 (2017).
A. Ashkenazi, C. F. Bento, T. Ricketts, et al., “Polyglutamine tracts regulate autophagy,” Autophagy, 13, No. 9, 1613–1614 (2017).
W. Y. Yau, E. O’Connor, R. Sullivan, et al., “DNA repair in trinucleotide repeat ataxias,” FEBS J., 285, No. 19, 3669–3682 (2018).
D. R. Scoles, P. Meera, M. D. Schneider, et al., “Antisense oligonucleotide therapy for spinocerebellar ataxia type 2,” Nature, 544, No. 7650, 362–366 (2017).
H. A. G. Teive, C. H. F. Camargo, and R. P. Munhoz, “Antisense oligonucleotide therapy for spinocerebellar ataxias: Good news for terrible diseases,” Mov. Disord. Clin. Pract., 5, 4, 400–403 (2018).
Y. A. Tsai, R. S. Liu, J. F. Lirng, et al., “Treatment of spinocerebellar ataxia with mesenchymal stem cells: A Phase I/IIa Clinical Study,” Cell Transplant., 26, No. 3, 503–512 (2017).
S. Romano, G. Coarelli, C. Marcotulli, et al., “Riluzole in patients with hereditary cerebellar ataxia: A randomised, double-blind, placebo- controlled trial,” Lancet Neurol., 14, No. 10, 985–991 (2015).
J. Liu, T. S. Tang, H. Tu, et al., “Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2,” J. Neurosci., 29, No. 29, 9148–9162 (2009).
A. W. Kasumu, X. Liang, P. Egorova, et al., “Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar Purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice,” J. Neurosci., 32, No. 37, 12786–12796 (2012).
D. D. Bushart, R. Chopra, V. Singh, et al., “Targeting potassium channels to treat cerebellar ataxia,” Ann. Clin. Transl. Neurol., 5, No. 3, 297–314 (2018).
S. Gispert, R. Twells, G. Orozco, et al., “Chromosomal assignment of the second locus for autosomal dominant cerebellar ataxia (SCA2) to chromosome 12q23-24.1,” Nat. Genet., 4, No. 3, 295–299 (1993).
M. Fernandez, M. E. McClain, R. A. Martinez, et al., “Late-onset SCA2: 33 CAG repeats are suffi cient to cause disease,” Neurology, 55, No. 4, 569–572 (2000).
S. M. Pulst, “The complex structure of ATXN2 genetic variation,” Neurol. Genet., 4, No. 6, e299 (2018).
L. E. Almaguer-Mederos, J. M. L. Mesa, Y. Gonzalez-Zaldivar, et al., “Factors associated with ATXN2 CAG/CAA repeat intergenerational instability in spinocerebellar ataxia type 2,” Clin. Genet., 94, No. 3–4, 346–350 (2018).
L. S. Sena, R. M. Castilhos, E. P. Mattos, et al., “Selective forces related to spinocerebellar ataxia type 2,” Cerebellum, 18, No. 2, 188–194 (2019).
S. van de Loo, F. Eich, D. Nonis, et al., “Ataxin-2 associates with rough endoplasmic reticulum,” Exp. Neurol., 215, No. 1, 110–118 (2009).
T. R. Kiehl, A. Nechiporuk, K. P. Figueroa, et al., “Generation and characterization of Sca2 (ataxin-2) knockout mice,” Biochem. Biophys. Res. Commun, 339, No. 1, 17–24 (2006).
I. Lastres-Becker, S. Brodesser, D. Lutjohann, et al., “Insulin receptor and lipid metabolism pathology in ataxin-2 knock-out mice,” Hum. Mol. Genet., 17, No. 10, 1465–1481 (2008).
M. Pfeffer, S. Gispert, G. Auburger, et al., “Impact of ataxin-2 knock out on circadian locomotor behavior and PER immunoreaction in the SCN of mice,” Chronobiol. Int., 34, No. 1, 129–137 (2017).
C. Lim and R. Allada, “ATAXIN-2 activates PERIOD translation to sustain circadian rhythms in Drosophila,” Science, 340, No. 6134, 875–879 (2013).
Y. Zhang, J. Ling, C. Yuan, et al., “A role for Drosophila ATX2 in activation of PER translation and circadian behavior,” Science, 340, No. 6134, 879–882 (2013).
K. Seidel, S. Siswanto, M. Fredrich, et al., “On the distribution of intranuclear and cytoplasmic aggregates in the brainstem of patients with spinocerebellar ataxia type 2 and 3,” Brain Pathol., 27, No. 3, 345–355 (2017).
N. S. Lim, G. Kozlov, T. C. Chang, et al., “Comparative peptide binding studies of the PABC domains from the ubiquitin-protein isopeptide ligase HYD and poly(A)-binding protein. Implications for HYD function,” J. Biol. Chem., 281, No. 20, 14,376–14,382 (2006).
I. Lastres-Becker, D. Nonis, F. Eich, et al., “Mammalian ataxin-2 modulates translation control at the pre-initiation complex via PI3K/ mTOR and is induced by starvation,” Biochim. Biophys. Acta, 1862, No. 9, 1558–1569 (2016).
U. Nonhoff, M. Ralser, F. Welzel, et al., “Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules,” Mol. Biol. Cell., 18, No. 4, 1385–1396 (2007).
B. Bakthavachalu, J. Huelsmeier, I. P. Sudhakaran, et al., “RNPgranule assembly via ataxin-2 disordered domains is required for long-term memory and neurodegeneration,” Neuron, 98, No. 4, 754– 766 e4 (2018).
L. A. Ostrowski, A. C. Hall, K. J. Szafranski, et al., “Conserved Pbp1/Ataxin-2 regulates retrotransposon activity and connects polyglutamine expansion-driven protein aggregation to lifespan-controlling rDNA repeats,” Commun. Biol., 1, 187 (2018).
N. E. Sen, J. Drost, S. Gispert, et al., “Search for SCA2 blood RNA biomarkers highlights Ataxin-2 as strong modifi er of the mitochondrial factor PINK1 levels,” Neurobiol. Dis., 96, 115–126 (2016).
P. P. Li, X. Sun, G. Xia, et al., “ATXN2-AS, a gene antisense to ATXN2, is associated with spinocerebellar ataxia type 2 and amyotrophic lateral sclerosis,” Ann. Neurol., 80, No. 4, 600–615 (2016).
M. Fittschen, I. Lastres-Becker, M. V. Halbach, et al., “Genetic ablation of ataxin-2 increases several global translation factors in their transcript abundance but decreases translation rate,” Neurogenetics, 16, No. 3, 181–192 (2015).
D. Meierhofer, M. Halbach, N. E. Sen, et al., “Ataxin-2 (Atxn2)- knock-out mice show branched chain amino acids and fatty acids pathway alterations,” Mol. Cell. Proteomics, 15, No. 5, 1728–39 (2016).
M. V. Halbach, S. Gispert, T. Stehning, et al., “Atxn2 Knockout and CAG42-knock-in cerebellum shows similarly dysregulated expression in calcium homeostasis pathway,” Cerebellum, 16, No. 1, 68–81 (2017).
E. D. Louis, S. H. Kuo, W. J. Tate, et al., “Heterotopic Purkinje cells: a comparative postmortem study of essential tremor and spinocerebellar ataxias 1, 2, 3, and 6,” Cerebellum, 17, No. 2, 104–110 (2018).
E. A. R. Nibbeling, A. Duarri, C. C. Verschuuren-Bemelmans, et al., “Exome sequencing and network analysis identifies shared mechanisms underlying spinocerebellar ataxia,” Brain, 140, No. 11, 2860– 2878 (2017).
B. E. Aboulhoda and S. S. Hassan, “Effect of prenatal tramadol on postnatal cerebellar development: Role of oxidative stress,” J. Chem. Neuroanat., 94, 102–118 (2018).
S. Squadrone, P. Brizio, C. Mancini, et al., “Altered homeostasis of trace elements in the blood of SCA2 patients,” J. Trace Elem. Med. Biol., 47, 111–114 (2018).
M. Guevara-Garcia, L. Gil-del Valle, L. Velasquez-Perez, and J. C. Garcia-Rodriguez, “Oxidative stress as a cofactor in spinocerebellar ataxia type 2,” Redox Rep., 17, No. 2, 84–89 (2012).
D. Almaguer-Gotay, L. E. Almaguer-Mederos, R. Aguilera-Rodriguez, et al., “Spinocerebellar ataxia type 2 is associated with the extracellular loss of superoxide dismutase but not catalase activity,” Front. Neurol., 8, 276 (2017).
L. E. Almaguer-Mederos, D. Almaguer-Gotay, R. Aguilera-Rodriguez, et al., “Association of glutathione S-transferase omega polymorphism and spinocerebellar ataxia type 2,” J. Neurol. Sci, 372, 324–328 (2017).
T. L. Monte, F. S. Pereira, E. D. R. Reckziegel, et al., “Neurological phenotypes in spinocerebellar ataxia type 2: Role of mitochondrial polymorphism A10398G and other risk factors,” Parkinsonism Relat. Disord., 42, 54–60 (2017).
H. Hamzeiy, D. Savas, C. Tunca, et al., “Elevated global DNA methylation is not exclusive to amyotrophic lateral sclerosis and is also observed in spinocerebellar ataxia types 1 and 2,” Neurodeg. Dis., 18, No. 1, 38–48 (2018).
C. Wilke, F. Bender, S. N. Hayer, et al., “Serum neurofi lament light is increased in multiple system atrophy of cerebellar type and in repeat- expansion spinocerebellar ataxias: A pilot study,” J. Neurol., 265, No. 7, 1618–1624 (2018).
D. P. Huynh, K. Figueroa, N. Hoang, and S. M. Pulst, “Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human,” Nat. Genet., 26, No. 1, 44–50 (2000).
A. W. Kasumu, C. Hougaard, F. Rode, et al., “Selective positive modulator of calcium-activated potassium channels exerts benefi cial effects in a mouse model of spinocerebellar ataxia type 2,” Chem. Biol., 19, No. 10, 1340–1353 (2012).
P. A. Egorova, O. A. Zakharova, O. L. Vlasova, and I. B. Bezprozvanny, “In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model,” J. Neurophysiol., 115, No. 6, 2840–2851 (2016).
P. A. Egorova, A. V. Gavrilova, and I. B. Bezprozvanny, “In vivo analysis of the climbing fiber-Purkinje cell circuit in SCA2-58Q transgenic mouse model,” Cerebellum, 17, No. 5, 590–600 (2018).
J. Aguiar, J. Fernandez, A. Aguilar, et al., “Ubiquitous expression of human SCA2 gene under the regulation of the SCA2 self promoter cause specifi c Purkinje cell degeneration in transgenic mice,” Neurosci. Lett., 392, No. 3, 202–206 (2006).
E. Damrath, M. V. Heck, S. Gispert, et al., “ATXN2-CAG42 sequesters PABPC1 into insolubility and induces FBXW8 in cerebellum of old ataxic knock-in mice,” PLoS Genetics, 8, No. 8, e1002920 (2012).
S. T. Hansen, P. Meera, T. S. Otis, and S. M. Pulst, “Changes in Purkinje cell fi ring and gene expression precede behavioral pathology in a mouse model of SCA2,” Hum. Mol. Genet., 22, No. 2, 271– 283 (2013).
L. T. Pflieger, W. Dansithong, S. Paul, et al., “Gene co-expression network analysis for identifying modules and functionally enriched pathways in SCA2,” Hum. Mol. Genet., 26, No. 16, 3069–3080 (2017).
W. Dansithong, S. Paul, K. P. Figueroa, et al., “Ataxin-2 regulates RGS8 translation in a new BAC-SCA2 transgenic mouse model,” PLoS Genetics, 11, No. 4, e1005182 (2015).
C. Y. Chuang, C. C. Yang, B. W. Soong, et al., “Modeling spinocerebellar ataxias 2 and 3 with iPSCs reveals a role for glutamate in disease pathology,” Sci. Rep., 9, No. 1, 1166 (2019).
A. G. Marthaler, B. Schmid, A. Tubsuwan, et al., “Generation of spinocerebellar ataxia type 2 patient-derived iPSC line H196,” Stem Cell. Res., 16, No. 1, 199–201 (2016).
J. A. Maguire, A. L. Gagne, P. Gonzalez-Alegre, et al., “Generation of spinocerebellar ataxia type 2 induced pluripotent stem cell lines, CHOPi002-A and CHOPi003-A, from patients with abnormal CAG repeats in the coding region of the ATXN2 gene,” Stem Cell. Res., 34, 101361 (2019).
T. W. Todd and J. Lim, “Aggregation formation in the polyglutamine diseases: Protection at a cost?” Mol. Cells, 36, No. 3, 185–194 (2013).
S. Koyano, S. Yagishita, Y. Kuroiwa, et al., “Neuropathological staging of spinocerebellar ataxia type 2 by semiquantitative 1C2-positive neuron typing. Nuclear translocation of cytoplasmic 1C2 underlies disease progression of spinocerebellar ataxia type 2,” Brain Pathol., 24, No. 6, 599–606 (2014).
M. Ueda, S. Li, M. Itoh, et al., “Polyglutamine expansion disturbs the endoplasmic reticulum formation, leading to caspase-7 activation through Bax,” Biochem. Biophys. Res. Commun, 443, No. 4, 1232– 1238 (2014).
N. Cornelius, J. H. Wardman, I. P. Hargreaves, et al., “Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 (SCA2) patient fi broblasts: Effect of coenzyme Q10 supplementation on these parameters,” Mitochondrion, 34, 103–114 (2017).
R. Y. Lo, K. P. Figueroa, S. M. Pulst, et al., “Coenzyme Q10 and spinocerebellar ataxias,” Mov. Disord., 30, No. 2, 214–220 (2015).
A. S. Brown, P. Meera, B. Altindag, et al., “MTSS1/Src family kinase dysregulation underlies multiple inherited ataxias,” Proc. Natl. Acad. Sci. USA, 115, No. 52, E12407–E12416 (2018).
D. S. Verbeek, J. Goedhart, L. Bruinsma, et al., “PKC gamma mutations in spinocerebellar ataxia type 14 affect C1 domain accessibility and kinase activity leading to aberrant MAPK signaling,” J. Cell Sci., 121, No. 14, 2339–2349 (2008).
E. Shimobayashi and J. P. Kapfhammer, “Calcium signaling, PKC gamma, IP3R1 and CAR8 link spinocerebellar ataxias and Purkinje cell dendritic development,” Curr. Neuropharmacol., 16, No. 2, 151–159 (2018).
R. Chopra, A. H. Wasserman, S. M. Pulst, et al., “Protein kinase C activity is a protective modifi er of Purkinje neuron degeneration in cerebellar ataxia,” Hum. Mol. Genet., 27, No. 8, 1396–1410 (2018).
M. K. Meffert, J. M. Chang, B. J. Wiltgen, et al., “NF-kappa B functions in synaptic signaling and behavior,” Nat. Neurosci., 6, No. 10, 1072–1078 (2003).
A. Ferro, W. Qu, A. Lukowicz, et al., “Inhibition of NF-kappaB signaling in IKKbetaF/F;LysM Cre mice causes motor deficits but does not alter pathogenesis of spinocerebellar ataxia type 1,” PLoS One, 13, No. 7, e0200013 (2018).
Y. X. Li, O. C. M. Sibon, and P. F. Dijkers, “Inhibition of NF-kappaB in astrocytes is sufficient to delay neurodegeneration induced by proteotoxicity in neurons,” J. Neuroinflammation, 15, No. 1, 261 (2018).
B. Alberts, Molecular Biology of the Cell, Garland Science, New York (2002), 4th ed.
M. Huang and D. S. Verbeek, “Why do so many genetic insults lead to Purkinje cell degeneration and spinocerebellar ataxia?” Neurosci. Lett., 688, 49–57 (2019).
B. Schwaller, M. Meyer, and S. Schiffmann, “’New’ functions for ‘old’ proteins: The role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice,” Cerebellum, 1, No. 4, 241–258 (2002).
L. Kreiner, C. J. Christel, M. Benveniste, et al., “Compensatory regulation of Cav2.1 Ca2+ channels in cerebellar Purkinje neurons lacking parvalbumin and calbindin D-28k,” J. Neurophysiol., 103, No. 1, 371–381 (2010).
M. Kano, H. Nakayama, K. Hashimoto, et al., “Calcium-dependent regulation of climbing fi bre synapse elimination during postnatal cerebellar development,” J. Physiol., 591, No. 13, 3151–3158 (2013).
J. A. Barnes, B. A. Ebner, L. A. Duvick, et al., “Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice,” J. Neurosci., 31, No. 36, 12778–12789 (2011).
C. Long, C. E. Grueter, K. Song, et al., “Ataxia and Purkinje cell degeneration in mice lacking the C-AMTA1 transcription factor,” Proc. Natl. Acad. Sci. USA, 111, No. 31, 11521–11526 (2014).
S. Y. Kawaguchi and T. Hirano, “Gating of long-term depression by Ca2+/calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells,” J. Physiol., 591, No. 7, 1707– 1730 (2013).
K. Fukumitsu, T. Hatsukano, A. Yoshimura, et al., “Mitochondrial fission protein Drp1 regulates mitochondrial transport and dendritic arborization in cerebellar Purkinje cells,” Mol. Cell. Neurosci., 71, 56–65 (2016).
D. Di Bella, F. Lazzaro, A. Brusco, et al., “Mutations in the mitochondrial protease gene AFG3L2 cause dominant hereditary ataxia SCA28,” Nat. Genet., 42, No. 4, 313–321 (2010).
E. R. Kandel, Principles of Neural Science, A. L. H. Sydor (ed.), The McGraw-Hill Companies, USA (2013).
D. W. Indriati, N. Kamasawa, K. Matsui, et al., “Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: Somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels,” J. Neurosci., 33, No. 8, 3668–3678 (2013).
C. Piochon, C. Levenes, G. Ohtsuki, and C. Hansel, “Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum,” J. Neurosci., 30, No. 45, 15,330–15,335 (2010).
R. Crupi, D. Impellizzeri, and S. Cuzzocrea, “Role of metabotropic glutamate receptors in neurological disorders,” Front. Mol. Neurosci., 12, 20 (2019).
H. Hirai and M. Kano, “Type 1 metabotropic glutamate receptor and its signaling molecules as therapeutic targets for the treatment of cerebellar disorders,” Curr. Opin. Pharmacol., 38, 51–58 (2018).
H. G. Serra, L. Duvick, T. Zu, et al., “RORalpha-mediated Purkinje cell development determines disease severity in adult SCA1 mice,” 127, No. 4, 697–708 (2006).
D. A. Gold, S. H. Baek, N. J. Schork, et al., “RORalpha coordinates reciprocal signaling in cerebellar development through sonic hedgehog and calcium-dependent pathways,” Neuron, 40, No. 6, 1119– 1131 (2003).
H. T. Orr, “SCA1-phosphorylation, a regulator of Ataxin-1 function and pathogenesis,” Prog. Neurobiol., 99, No. 3, 179–185 (2012).
P. Meera, S. Pulst, and T. Otis, “A positive feedback loop linking enhanced mGluR function and basal calcium in spinocerebellar ataxia type 2,” eLife, 6 (2017).
A. J. Collins, R. N. Foley, C. Herzog, et al., “US Renal Data System 2012 Ann Data Report,” Am. J. Kidney Dis., 61, No. 1, Suppl. 1, A7, e1-476 (2013).
P. Meera, S. M. Pulst, and T. S. Otis, “Cellular and circuit mechanisms underlying spinocerebellar ataxias,” J. Physiol., 594, No. 16, 4653–4660 (2016).
P. Grumati and I. Dikic, “Ubiquitin signaling and autophagy,” J. Biol. Chem., 293, No. 15, 5404–5413 (2018).
N. Jatana, D. B. Ascher, D. E. V. Pires, et al., “Human LC3 and GABARAP subfamily members achieve functional specifi city via specifi c structural modulations,” Autophagy, 14, 1–17 (2019).
G. Puorro, A. Marsili, F. Sapone, et al., “Peripheral markers of autophagy in polyglutamine diseases,” Neurol. Sci., 39, No. 1, 149–152 (2018).
S. Paul, W. Dansithong, K. P. Figueroa, et al., “Staufen1 links RNA stress granules and autophagy in a model of neurodegeneration,” Nat. Commun., 9, No. 1, 3648 (2018).
A. Ferro, C. Sheeler, J. G. Rosa, and M. Cvetanovic, “Role of microglia in ataxias,” J. Mol. Biol., (2019).
M. S. Thion, D. Low, A. Silvin, et al., “Microbiome influences prenatal and adult microglia in a sex-specific manner,” Cell, 172, No. 3, 500–516 e16 (2018).
H. Nakayama, M. Abe, C. Morimoto, et al., “Microglia permit climbing fi ber elimination by promoting GABAergic inhibition in the developing cerebellum,” Nat. Commun., 9, No. 1, 2830 (2018).
B. A. Ebner, M. A. Ingram, J. A. Barnes, et al., “Purkinje cell ataxin- 1 modulates climbing fi ber synaptic input in developing and adult mouse cerebellum,” J. Neurosci., 33, No. 13, 5806–5820 (2013).
M. Cvetanovic, M. Ingram, H. Orr, and P. Opal, “Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1,” Neuroscience, 289, 289–299 (2015).
W. Qu, A. Johnson, J. H. Kim, et al., “Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice,” J. Neuroinflammation, 14, No. 1, 107 (2017).
R. Llinas and M. Sugimori, “Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices,” J. Physiol., 305, 197–213 (1980).
R. Llinas and M. Sugimori, “Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices,” J. Physiol., 305, 171–195 (1980).
I. M. Raman and B. P. Bean, “Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons,” J. Neurosci., 17, No. 12, 4517–4526 (1997).
I. M. Raman and B. P. Bean, “Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons,” J. Neurosci., 19, No. 5, 1663–1674 (1999).
S. C. Nam and P. E. Hockberger, “Analysis of spontaneous electrical activity in cerebellar Purkinje cells acutely isolated from postnatal rats,” J. Neurobiol., 33, No. 1, 18–32 (1997).
M. Womack and K. Khodakhah, “Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons,” J. Neurosci., 22, No. 24, 10603–10612 (2002).
S. L. Smith and T. S. Otis, “Persistent changes in spontaneous fi ring of Purkinje neurons triggered by the nitric oxide signaling caSCAde,” J. Neurosci., 23, No. 2, 367–372 (2003).
C. I. De Zeeuw, F. E. Hoebeek, L. W. Bosman, et al., “Spatiotemporal firing patterns in the cerebellum,” Nat. Rev. Neurosci., 12, No. 6, 327–344 (2011).
F. E. Hoebeek, J. S. Stahl, A. M. van Alphen, et al., “Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control,” Neuron, 45, No. 6, 953–965 (2005).
K. Alvina and K. Khodakhah, “The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia,” J. Neurosci., 30, No. 21, 7258–7568 (2010).
J. M. Dell’Orco, A. H. Wasserman, R. Chopra, et al., “Neuronal atrophy early in degenerative ataxia is a compensatory mechanism to regulate membrane excitability,” J. Neurosci., 35, No. 32, 11292– 11307 (2015).
M. D. Mark, M. Krause, H. J. Boele, et al., “Spinocerebellar ataxia type 6 protein aggregates cause defi cits in motor learning and cerebellar plasticity,” J. Neurosci., 35, No. 23, 8882–8895 (2015).
V. G. Shakkottai, M. do Carmo Costa, J. M. Dell’Orco, et al., “Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3,” J. Neurosci., 31, No. 36, 13002–13014 (2011).
J. T. Walter, K. Alvina, M. D. Womack, et al., “Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia,” Nat. Neurosci., 9, No. 3, 389–397 (2006).
R. Chopra and V. G. Shakkottai, “Translating cerebellar Purkinje neuron physiology to progress in dominantly inherited ataxia,” Future Neurol., 9, No. 2, 187–196 (2014).
J. M. Dell’Orco, S. M. Pulst, and V. G. Shakkottai, “Potassium channel dysfunction underlies Purkinje neuron spiking abnormalities in spinocerebellar ataxia type 2,” Hum. Mol. Genet., 26, No. 20, 3935– 3945 (2017).
D. D. Bushart and V. G. Shakkottai, “Ion channel dysfunction in cerebellar ataxia,” Neurosci. Lett., 688, 41–48 (2019).
M. Coutelier, G. Coarelli, M. L. Monin, et al., “A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies,” Brain, 140, No. 6, 1579–1594 (2017).
J. M. Jones, L. Dionne, J. Dell’Orco, et al., “Single amino acid deletion in transmembrane segment D4S6 of sodium channel Scn8a (Nav1.6) in a mouse mutant with a chronic movement disorder,” Neurobiol. Dis., 89, 36–45 (2016).
K. H. Lee, P. J. Mathews, A. M. Reeves, et al., “Circuit mechanisms underlying motor memory formation in the cerebellum,” Neuron, 86, No. 2, 529–540 (2015).
E. J. Lang, R. Apps, F. Bengtsson, et al., “The roles of the olivocerebellar pathway in motor learning and motor control. A consensus paper,” Cerebellum, 16, No. 1, 230–252 (2017).
S. H. Kuo, C. Y. Lin, J. Wang, et al., “Climbing fi ber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases,” Acta Neuropathol., 133, No. 1, 121–138 (2017).
A. Burroughs, A. K. Wise, J. Xiao, et al., “The dynamic relationship between cerebellar Purkinje cell simple spikes and the spikelet number of complex spikes,” J. Physiol., 595, No. 1, 283–299 (2017).
J. T. Davie, B. A. Clark, and M. Hausser, “The origin of the complex spike in cerebellar Purkinje cells,” J. Neurosci., 28, No. 30, 7599– 7609 (2008).
T. V. Karelina and R. A. Grigor’ian, “Effect of harmaline of the complex spike waveform and depression time in cerebellar Purkinje cell discharge in rat postnatal ontogenesis,” Zh. Evol. Biokhim. Fiziol., 46, No. 3, 218–224 (2010).
W. M. C. van Roon-Mom, R. A. C. Roos, and S. T. de Bot, “Dosedependent lowering of mutant huntingtin using antisense oligonucleotides in Huntington disease patients,” Nucleic Acid Ther., 28, No. 2, 59–62 (2018).
C. Rinaldi and M. J. A. Wood, “Antisense oligonucleotides: The next frontier for treatment of neurological disorders,” Nat. Dev. Neurol., 14, No. 1, 9–21 (2018).
R. Volkman and D. Offen, “Concise review: mesenchymal stem cells in neurodegenerative diseases,” Stem Cells, 35, No. 8, 1867–1880 (2017).
Y. K. Chang, M. H. Chen, Y. H. Chiang, et al., “Mesenchymal stem cell transplantation ameliorates motor function deterioration of spinocerebellar ataxia by rescuing cerebellar Purkinje cells,” J. Biomed. Sci., 18, 54 (2011).
L. T. Cho, A. J. Alexandrou, R. Torella, et al., “An Intracellular allosteric modulator binding pocket in SK2 ion channels is shared by multiple chemotypes,” Structure, 26, No. 4, 533-544 e3 (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 105, No. 11, pp. 1349–1372, November, 2019.
Rights and permissions
About this article
Cite this article
Egorova, P.A., Bezprozvanny, I.B. New Approaches in Studies of the Molecular Pathogenesis of Type 2 Spinocerebellar Ataxia. Neurosci Behav Physi 50, 938–951 (2020). https://doi.org/10.1007/s11055-020-00988-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00988-x