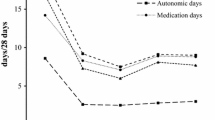
Objectives. To assess the value of using different treatment schemes in chronic migraine by comparing clinical results and the economic burdens of disease in real clinical practice. Materials and methods. The study included 66 patients attending the Academician Aleksandr Vein Headache and Autonomic Disorders Clinic: 60 women and six men aged 28–51 years with diagnoses of chronic migraine. The patients were divided into three groups: group 1 (n = 22) consisted of patients who received three months of oral prophylactic therapy with topiramate at doses of up to 100 mg/day; patients of group 2 (n = 20) received 12 sessions of acupuncture with three procedures per week; patients of group 3 (n = 24) received injections of botulinum toxin type A (Botox, BTA) at a dose of 155–195 U. The observation period was three months. Treatment efficacy was assessed using the following methods: clinical-neurological assessment, the Headache Impact Test HIT-6 questionnaire, and a subjective points questionnaire assessment for treatment satisfaction and tolerance. Results. BTA was the most effective of the three treatment methods studied in patients with chronic migraine. As compared with oral prophylactic therapy and acupuncture, BTA produced the fastest and strongest actions on the frequency of headache, promoting regression of chronic migraine and recovery of the episodic nature of headache (the numbers of headache days in group 1, 2, and 3 were 16.1 ± 0.1, 18.0 ± 0.02, and 13.9 ± 0.3, respectively, at one month). BTA also produced significantly faster and more effective recovery of quality of life and was better tolerated (good in 51%, 75%, and 85% in groups 1, 2, and 3, respectively; satisfactory in 35%, 25%, and 15% in groups 1, 2, and 3, respectively; poor in 14% in the oral prophylaxis group). Most patients in the BTA group achieved satisfactory treatment results more quickly. Despite the greater direct costs as compared with topiramate, the direct costs associated with the use of BTA (29931.51 and 32085.87 rubles, respectively, the predicted cost per non-headache day in the BTA group was the lowest, at 652.15 rubles (692.86 and 1017.60 rubles in the oral prophylaxis and acupuncture groups, respectively). Conclusions. The efficacy and cost data obtained here for the different methods of prophylaxis of chronic migraine may help specialists and patients select the most optimal therapeutic approaches.
Similar content being viewed by others
References
Headache Classification Committee of the International Headache Society (IHS), “The International Classification of Headache Disorders: 3rd edition,” Cephalalgia, 33, No. 9, 629–808 (2013), https://doi.org/10.1177/0333102413485658.
I. Ayzenberg, Z. Katsarava, A. Sborowski, et al., “The prevalence of primary headache disorders in Russia: A country wide survey,” Cephalalgia, 32, 373–381 (2012), https://doi.org/10.1177/0333102412438977.
D. W. Dodick, C. C. Turkel, R. E. DeGryse, et al., “Onabotulinum toxin A for treatment of chronic migraine: pooled results from the double-blind, randomized, placeb-ocontrolled phases of the PREEMPT clinical program,” Headache, 50, No. 6, 921–936 (2010), https://doi.org/10.1111/j.1526-4610.2010.01678.x.
M. V. Naprienko, L. V. Smekalkina, and E. A. Surnova, “Efficacy of different doses of botox in treatment of chronic migraine,” Zh. Nevrol. Psikhiat., 117, No. 8, 44–48 (2017), https://doi.org/10.17116/jnevro20171178144-48.
L. M. Bloudek, M. Stokes, D. C. Buse, et al., “Cost of healthcare for patients with migraine in fi ve European countries: results from the International Burden of Migraine Study (IBMS),” J. Headache Pain, 13, No. 5, 361–378 (2012), https://doi.org/10.1007/s10194-012-0460-7.
J. Berg, “Economic evidence in migraine and other headaches: a review,” Eur. J. Health Econ., 5, Supplement 1, 43–54 (2004), https://doi.org/10.1007/s10198-005-0288-z.
M. Linde, T. J. Steiner, and D. Chisholm, “Cost-effectiveness analysis of interventions for migraine in four low- and middle-income countries,” J. Headache Pain, 16, 15 (2015), https://doi.org/10.1186/s10194-015-0496-6.
T. T. Glembotskaya and O. V. Kozub, “Pharmacoeconomic assessment of the ‘burden’ of migraine in the Russian Federation,” Klin. Farmakol. Terapiya, 2, 83–86 (2013).
V. V. Osipova, E. G. Filatova, A. R. Artemenko, et al., “Diagnosis and treatment of migraine: Recommendations of Russian experts,” Zh. Nevrol. Psikhiat., 117, No. 1–2, 28–42 (2017), https://doi.org/10.17116/jnevro20171171228-42.
M. Khalil, H. W. Zafar, V. Quarshie, and F. Ahmed, “Prospective analysis of the use of Onabotulinumtoxin A (BOTOX) in the treatment of chronic migraine; real- life data in 254 patients from Hull, UK,” J. Headache Pain, 15, 54 (2014), https://doi.org/10.1186/1129-2377-15-54.
E. Cernuda-Morollón, C. Ramon, P. Martínez-Camblor, et al., “Onabotulinumtoxin A decreases interictal CGRP plasma levels in patients with chronic migraine,” Pain, 156, No. 5, 820–824 (2015), https://doi.org/10.1097/j.pain.0000000000000119.
B. Davies, C. Gaul, P. Martelletti, et al., “Real-life use of onabotulinumtoxin A for symptom relief in patients with chronic migraine: REPOSE study methodology and baseline data,” J. Headache Pain, 18, No. 1, 93 (2017), https://doi.org/10.1186/s10194-017-0802-6.
A. M. Blumenfeld, S. K. Aurora, K. Laranjo, and S. Papapetropoulos, “Unmet clinical needs in chronic migraine: Rationale for study and design of COMPEL, an open-label, multicenter study of the long-term efficacy, safety, and tolerability of onabotulinumtoxin A for headache prophylaxis in adults with chronic migraine,” BMC Neurol., 15, 100 (2015), https://doi.org/10.1186/s12883-015-0353-x.
M. V. Naprienko and L. V. Smekalkina, “Strategies for improving treatment efficacy in chronic migraine,” Zh. Nevrol. Psikhiat., 115, No. 12, 70–73 (2015), https://doi.org/10.17116/jnevro20171178144-48.
V. V. Osipova, Yu. E. Azimova, G. R. Tabeeva, et al., “Diagnosis of headache in Russian and post-Soviet bloc countries: the state of the problem and ways to solve it,” Ann. Klin. Eksperim. Nevrol., 2, 16–16. (2012), https://cyberleninka.ru/article/n/diagnostika-golovnyhboley-v-rossii-i-stranah-postsovetskogo-prostranstva-sostoyanie-problemyi-puti-ee-resheniya.
K. V. Таtаrinova and A. R. Аrtemenko, “Influences of the clinical manifestations of migraine, depression, and sleep disorders on the quality of life of patients with chronic migraine,” Nervno-Mysh. Bol., 7, No. 1, 43–53 (2017), https://doi.org/10.17650/2222-8721-2017-7-1-43-53.
C. P. Yang, M. H. Chang, P. E. Liu, et al., “Acupuncture versus topiramate in chronic migraine prophylaxis: a randomized clinical trial,” Cephalalgia, 31, No. 15, 1510–1521 (2011), https://doi.org/10.1177/0333102411420585.
N. T. Mathew and S. F. Jaffri, “A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study,” Headache, 49, No. 10, 1466–1478 (2009), https://doi.org/10.1111/j.1526-4610.2009.01566.x.
B. Naderinabi, A. Saberi, M. Hashemi, et al., “Acupuncture and botulinum toxin A injection in the treatment of chronic migraine: A randomized controlled study,” Caspian J. Intern. Med., 8, No. 3, 196–204 (2017), https://doi.org/10.22088/cjim.8.3.196.
C. N. Homann, K. Suppan, K. Wenzel, et al., “East-west differences in the organization of botulinum toxin use in nine Central European countries,” Eur. J. Neurol., 10, No. 3, 213–219 (2003).
S. M. Schaefer, C. H. Gottschalk, and B. Jabbari, “Treatment of chronic migraine with focus on botulinum neurotoxins,” Toxins, 7, 2615–2628 (2015), https://doi.org/10.3390/toxins7072615.
S. K. Aurora, D. W. Dodick, H. C. Diener, et al., “Onabotulinumtoxina for chronic migraine: Efficacy, safety, and tolerability in patients who received all five treatment cycles in the preempt clinical program,” Acta Neurol. Scand., 129, No. 1, 61–70 (2014), https://doi.org/10.1111/ane.12171.
N. T. Mathew and S. F. Jaffri, “A double-blind comparison of onabotulinumtoxina (BOTOX) and topiramate (TOPAMAX) for the prophylactic treatment of chronic migraine: a pilot study,” Headache, 49, No. 10, 1466–1478 (2009), https://doi.org/10.1111/j.1526-4610.2009.01566.x.
M. Lanteri-Minet, “Economic burden and costs of chronic migraine,” Curr. Pain Headache Rep., 18, No. 1, 385 (2014), https://doi.org/10.1007/s11916-013-0385-0.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 119, No. 1, Iss. 1, pp. 31–37, January, 2019.
Rights and permissions
About this article
Cite this article
Naprienko, M.V., Smekalkina, L.V., Safonov, M.I. et al. The Burden of Migraine in Real Clinical Practice: Clinical and Economic Aspects. Neurosci Behav Physi 50, 20–26 (2020). https://doi.org/10.1007/s11055-019-00862-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00862-5