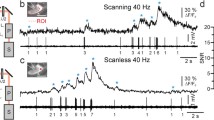
Widening and deepening our understanding of how the brain works requires constant improvements not only in methods of recording neuron activity, but also improvements in experimental approaches to activating individual cells and their compartments. Optogenetic stimulation methods using finely focused light to trigger the opening of the light-activated depolarizing cation channel rhodopsin-2 (ChR2) have become widely used in recent years. Current molecular biological methods provide for the genetic expression of ChR2 in different cell types, which, along with the ability to carry out electrophysiological experiments with reproducible patterns of activation and stable levels of ChR2 expression, have developed optogenetics into an effective method for gathering physiological data previously unavailable to conventional methods. We report here the use of local activation of axons using an optogenetic stimulation method. Experiments were performed in combination with recording the electrical activity of neurons using the patch-clamp method, as well as laser scanning confocal microscopy. Experiments used the transgenic mouse strain Thy1-ChR2-YFP, in which ChR2 is expressed in only a small proportion of pyramidal cells. Direct studies of the effects of functional activity in the proximal branches of pyramidal neuron axons in layer 5 of the visual cortex and hippocampal field CA1 on the shape and generation of action potentials were carried out. We also describe methodological advances and means of solving problems encountered in the optogenetic stimulation of the axons of pyramidal neurons in the central nervous system of mammals.
Similar content being viewed by others
References
Aseev, N. A., Nikitin, E. S., Roshchin, M. V., et al., “Biolistic delivery of voltage-dependent dyes into cells in living brain slices from mammals for optical recording of neuron activity,” Zh. Vyssh. Nerv. Deyat., 62, 100–107 (2012).
Bähner, F., Weiss, E. K., Birke, G., et al., “Cellular correlate of assembly formation in oscillating hippocampal networks in vitro,” Proc. Natl. Acad. Sci. USA, 108, No. 35, E607–E616 (2011).
Buzsáki, G., “Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning,” Hippocampus, 25, 1073–1188 (2015).
Buzsaki, G., “Hippocampal sharp waves: their origin and significance,” Brain Res., 398, No. 2, 242–252 (1986).
Buzsáki, G., “Two-stage model of memory trace formation: a role for “noisy” brain states,” Neuroscience, 31, 551–570 (1989).
Debanne, D. and Boudkkazi, S., “New insights in information processing in the axon,” in: New Aspects of Axonal Structure and Function
D. Feldmeyer and J. H. R. Lübke (eds.), Springer, Boston, MA (2010), pp. 55–83.
Foust, A. J., Yu, Y., Popovic, M., et al., “Somatic membrane potential and Kv1 channels control spike repolarization in cortical axon collaterals and presynaptic boutons,” J. Neurosci., 31, No. 43 15490–15498 (2011).
Groh, A., Meyer, H. S., Schmidt, E. F., et al., “Cell-type specific properties of pyramidal neurons in neocortex underlying a layout that is modifiable depending on the cortical area,” Cereb. Cortex, 20, No. 4, 826–836 (2010).
Grubb, M. S. and Burrone, J., “Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability,” Nature, 465, No. 7301, 1070–1074 (2010).
Kole, M. H., “First node of Ranvier facilitates high-frequency burst encoding,” Neuron, 71, No. 4, 671–682 (2011).
Kole, M. H., Letzkus, J. J., and Stuart, G. J., “Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy,” Neuron, 55, No. 4, 633–647 (2007).
Maier, N., Nimmrich, V., and Draguhn, A., “Cellular and network mechanisms underlying spontaneous sharp wave-ripple complexes in mouse hippocampal slices,” J. Physiol., 550, No. 3, 873–887 (2003).
Nikitin, E. S., Bal, N. V., Malyshev, A., et al., “Encoding of high frequencies improves with maturation of action potential generation in cultured neocortical neurons,” Front. Cell. Neurosci., 11, 28 (2017).
Nikitin, E. S., Malyshev, A. Yu., Balaban, P. M., and Volgushev, M. A., “Physiological aspects of the use of a the Hodgkin-Huxley model of action potential generation for neurons in invertebrates and vertebrates,” Zh. Vyssh. Nerv. Deyat., 3, 279–288 (2016).
Palmer, L. M. and Stuart, G. J., “Site of action potential initiation in layer 5 pyramidal neurons,” J. Neurosci., 26, No. 6, 1854–1863 (2006).
Popovic, M. A., Foust, A. J., McCormick, D. A., and Zecevic, D., “The spatio-temporal characteristics of action potential initiation in layer 5 pyramidal neurons: a voltage imaging study,” J. Physiol., 589, No. 17, 4167–4187 (2011).
Romand, S., Wang, Y., Toledo-Rodriguez, M., and Markram, H., “Morphological development of thick-tufted layer V pyramidal cells in the rat somatosensory cortex,” Front. Neuroanat., 5, 5 (2011).
Thome, C., Kelly, T., Yanez, A., et al., “Axon-carrying dendrites convey privileged synaptic input in hippocampal neurons,” Neuron, 83, No. 6, 1418–1430 (2014).
Vladimirov, N., Tu, Y., and Traub, R. D., “Synaptic gating at axonal branches, and sharp-wave ripples with replay: a simulation study,” Eur. J. Neurosci., 38, No. 10, 3435–3447 (2013).
Wang, G., Wyskiel, D. R., Yang, W., et al., “An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits,” Nat. Protoc., 10, No. 3, 397–412 (2015).
Yu, Y., Maureira, C., Liu, X., and McCormick, D., “P/Q and N channels control baseline and spike-triggered calcium levels in neocortical axons and synaptic boutons,” J. Neurosci., 30, No. 35, 11858–11 869 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 67, No. 5, pp. 101–108, September–October, 2017.
Rights and permissions
About this article
Cite this article
Nikitin, E.S., Roshchin, M.V., Ierusalimsky, V.N. et al. Optogenetic Stimulation of the Axons of Visual Cortex and Hippocampus Pyramidal Neurons in Living Brain Slices. Neurosci Behav Physi 49, 227–232 (2019). https://doi.org/10.1007/s11055-019-00719-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00719-x