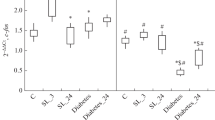
One of the complication of type 1 diabetes mellitus (DM1) is cognitive deficit, developing as a result of neurodegenerative changes in the brain. The aims of the present work were to study learning and spatial memory in rats with streptozotocin DM1 of different durations (1.5 and 6 months) and to investigate the activity of the adenylate cyclase signal system (ACSS) in the brain, this system being sensitive to serotonin and dopamine receptor agonists. Rats with DM1 for 1.5 months showed no impairment to spatial memory in a Morris water maze. When the duration of DM1 was increased to six months, spatial memory and learning ability decreased. Impaired regulation of adenylate cyclase by types 1 and 6 serotonin receptor antagonists and type 2 dopamine receptors was seen at both durations of DM1, indicating that these are primary in the development of cognitive deficit. Impairments to the ACSS can be regarded as key factors in the etiology and pathogenesis of cognitive dysfunctions in DM1. It is hypothesized that cognitive deficit develops only at the late stages of DM1, and results from impairments to the serotonin and dopamine signal systems of the brain.
Similar content being viewed by others
References
I. B. Sukhov, V. N. Shipilov, O. V. Chistyakova, et al., “Prolonged intranasal administration of insulin improves spatial memory in male rats with long-term diabetes mellitus,” Dokl. Akad. Nauk, 453, No. 5, 577–580 (2013).
A. O. Shpakov, “Functional state of biogenic amine- and acetylcholine-regulated brain signal systems in diabetes mellitus,” Tsitologiya, 54, No. 6, 459–468 (2012).
P. M. Abraham, K. P. Kuruvilla, J. Mathew, et al., “Alterations in hippocampal serotonergic and INSR function in streptozotocin induced diabetic rats exposed to stress: neuroprotective role of pyridoxine and Aegle marmelose,” J. Biomed. Sci., 17, 78 (2010).
E. Benito and A. Barco, “CREB’s control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models,” Trends Neurosci., 33, No. 5, 230–240 (2010).
G. J. Biessels and W. H. Gispen, “The impact of diabetes on cognition: what can be learned from rodent models?” Neurobiol. Aging, 26, Supplement 1, 36–41 (2005).
G. J. Biessels, A. Kamal, G. M. Ramakers, et al., “Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats,” Diabetes, 45, No. 9, 1259–1266 (1996).
G. J. Biessels, A. Kamal, I. J. Urban, et al., “Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment,” Brain Res., 800, No. 1, 125–135 (1998).
A. M. Brands, G. J. Biessels, E. H. de Haan, et al., “The effects of type 1 diabetes on cognitive performance: a meta-analysis,” Diabetes Care, 28, 726–735 (2005).
A. M. Brands, R. P. Kessels, R. P. Hoogma, et al., “Cognitive performance, psychological well-being, and brain magnetic resonance imaging in older patients with type 1 diabetes,” Diabetes, 55, 1800–1806 (2006).
O. V. Chistyakova, V. M. Bondareva, V. N. Shipilov, et al., “A positive effect of intranasal insulin on spatial memory in rats with neonatal diabetes mellitus,” Endocrinol. Studies, 1 (e16), 67–75 (2011).
E. Ezzeldin, W. A. Souror, T. El-Nahhas, et al., “Biochemical and neurotransmitters changes associated with tramadol in streptozotocin-induced diabetes in rats,” Biomed. Res. Int., Article ID 238780 (2014).
A. M. Jacobson, G. Musen, C. M. Ryan, et al., “Long-term effect of diabetes and its treatment on cognitive function,” New Engl. J. Med., 356, 1842–1852 (2007).
A. Kamal, G. J. Biessels, S. E. Duis, and W. H. Gispen, “Learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: interaction of diabetes and ageing,” Diabetologia, 43, No. 4, 500–506 (2000).
A. Kamal, G. J. Biessels, G. M. Ramakers, and W. Hendrik Gibsen, “The effect of short duration streptozotocin-induced diabetes mellitus on the late phase and threshold of long-term potentiation induction in the rat,” Brain Res., 1053, No. 1–2, 126–130 (2005).
M. Khelfaoui, F. Gambino, X. Houbaert, et al., “Lack of the presynaptic RhoGAP protein oligophrenin 1 leads to cognitive disabilities through dysregulation of the cAMP/PKA signalling pathway,” Philos. Trans. R. Soc. Lond. B Biol. Sci., 369, No. 1633, 20130160 (2013).
T. P. Kumar, S. Antony, G. Gireesh, et al., “Curcumin modulates dopaminergic receptor, CREB and phospholipase C gene expression in the cerebral cortex and cerebellum of streptozotocin induced diabetic rats,” J. Biomed. Sci., 17, No. 43, 1–11 (2010).
G. Malleret, R. Hen, J. L. Guillou, et al., “5-HT1B receptor knockout mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze,” J. Neurosci., 19, No. 14, 6157–6168 (1999).
A. Meneses, “5-HT system and cognition,” Neurosci. Biobehav. Rev., 23, No. 8, 1111–1125 (1999).
M. Nasehi, M. Jamshidi-Mehr, F. Khakpai, and M. R. Zarrindast, “Possible involvement of CAI 5-HT1B/1D and 5-HT2A/2B/2C receptors in harmaline-induced amnesia,” Pharmacol. Biochem. Behav., 125, 70–77 (2014).
C. R. Noguira, U. F. Machado, R. Curi, and A. R. Carpinelli, “Modulation of insulin secretion and 45Ca2+ efflux by dopamine in glucose-stimulated pancreatic islets,” Gen. Pharmacol., 25, 909–1016 (1994).
I. Papazoglou, F. Berthou, N. Vicaire, et al., “Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model,” Mol. Cell Endocrinol., 350, No. 1, 136–144 (2012).
G. Perez-Garcia and A. Meneses, “Memory formation, amnesia, improved memory and reversed amnesia: 5-HT role,” Behav. Brain Res., 195, No. 1, 17–29 (2008).
M. V. Puig, J. Rose, R. Schmidt, and N. Freund, “Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds,” Front Neural Circuits, 8, 93 (2014).
L. P. Reagan, “Diabetes as a chronic metabolic stressor: causes, consequences and clinical complications,” Exp. Neurol., 233, No. 1, 68–78 (2012).
R. Robinson, A. Krishnakumar, and C. S. Paulose, “Enhanced dopamine D1 and D2 receptor gene expression in the hippocampus of hypoglycaemic and diabetic rats,” Cell. Mol. Neurobiol., 29, No. 3, 365–372 (2009).
C. M. Ryan, M. O. Geckle, and T. J. Orchard, “Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications,” Diabetologia, 46, No. 7, 940–948 (2003).
M. Sandrini, G. Vitale, A. V. Vergoni, et al., “Streptozotocin-induced diabetes provokes changes in serotonin concentration and on 5-HT1A and 5-HT2 receptors in the rat brain,” Life Sci., 60, No. 16, 1393–1397 (1997).
A. Scheen, “Central nervous system: a conductor orchestrating metabolic regulations harmed by both hyperglycaemia and hypoglycaemia,” Diabetes Metab., 36, 31–38 (2010).
A. Shpakov, O. Chistyakova, K. Derkach, and V. Bondareva, “Hormonal signaling systems of the brain in diabetes mellitus,” in: Neurodegenerative Diseases, R. C.-C. Chang (ed.), Intech Open Access Publisher, Rijeka, Croatia (2011), pp. 349–386.
A. O. Shpakov, O. V. Chistyakova, K. V. Derkach, et al., “Intranasal insulin affects adenylyl cyclase system in rat tissues in neonatal diabetes,” Central Eur. J. Biol., 7, 33–47 (2012).
A. O. Shpakov, K. V. Derkach, O. V. Chistyakov, et al., “The brain adenylyl cyclase signaling system and cognitive functions in rats with neonatal diabetes under the influence of intranasal serotonin,” J. Metab. Syndr., 1, No. 2, 1–9 (2012).
A. O. Shpakov, E. A. Shpakova, I. I. Tarasenko, et al., “The peptides mimicking the third intracellular loop of 5-hydroxytryptamine receptors of the types 1B and 6 selectively activate G proteins and receptor-specifically inhibit serotonin signaling via the adenylyl cyclase system,” Int. J. Pept. Res. Ther., 16, 95–105 (2010).
H. Umegaki, J. Munoz, R. C. Meyer, et al., “Involvement of dopamine D(2) receptors in complex maze learning and acetylcholine release in ventral hippocampus of rats,” Neuroscience, 103, No. 1, 27–33 (2001).
M. A. Van Tilburg, C. C. McCaskill, J. D. Loane, et al., “Depressed mood is a factor in glycemic control in type 1 diabetes,” Psychosom. Med., 63, 551–555 (2001).
S. A. Wrighten, G. G. Piroli, C. A. Grillo, and L. P. Reagan, “A look inside the diabetic brain: Contributors to diabetes-induced brain aging,” Biochim. Biophys. Acta, 1792, No. 5, 444–453 (2009).
T. Yamato, Y. Misumi, S. Yamasaki, et al., “Diabetes mellitus decreases hippocampal release of neurotransmitters: an in vivo microdialysis study of awake, freely moving rats,” Diabet. Nutr. Metab., 17, 128–136 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 101, No. 3, pp. 279–290, March, 2015.
Rights and permissions
About this article
Cite this article
Sukhov, I.B., Chistyakova, O.V., Shipilov, V.N. et al. Spatial Memory and the Control of Adenylate Cyclase by Serotonin and Dopamine in the Brain in Rats with Streptozotocin Diabetes. Neurosci Behav Physi 46, 632–638 (2016). https://doi.org/10.1007/s11055-016-0289-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-016-0289-7