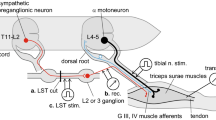
A comparative analysis of baseline activity, tetanic and post-tetanic increases and decreases in the frequencies of spinal cord motoneuron responses to high-frequency (50 and 100 Hz) stimulation (HFrS) of the flexor (G) and extensor (P) nerves of the hindlimb was performed in normal rats and rats with parathyroprivous (hypocalcemic) tetany. Experiments were run using on-line selection and programmed analysis of spike activity. Significant shifts in tetanic and post-tetanic motoneuron activity were recorded on days 3–7 and 21–22 of the development of acute and chronic tetanization respectively, without any significant change in baseline activity. Along with sharp increases in post-stimulus excitatory changes in activity in response to HFrS during the development of acute tetany, there was relative weakening in animals with chronic tetany. At the same time, there was weakening or complete disappearance of depressor reactions during the period of development of acute tetany with some degree of stabilization in animals with latent tetany. A cause-effect relationship was identified between motor disorders in parathyroid insufficiency on the one hand and impairments to the ratio of inhibitory/excitatory processes in spinal cord motoneurons on the other.
Similar content being viewed by others
References
P. G. Kostyuk, Calcium and Cellular Excitability, Nauka, Moscow (1986).
P. G. Kostyuk, E. A. Luk’yanets, A. S. Ter-Markosyan, and D. N. Khudaverdyan, “Parathyroid hormone stimulation of the calcium conductivity of nerve cells,” Neirofiziologiya, 22, No. 3, 373–380 (1990).
A. S. Ter-Markosyan, V. K. Lutsenko, D. N. Khudaverdyan, and N. N. Khlebnikova, “Transport of 45Ca2+ and 3H-GABA in nerve endings isolated from the cerebral cortex of rats with hyperparathyroidism,” Byull. Eksperim. Biol. Med., III, No. 10, 402–404 (1987).
D. N. Khudaverdyan, “Characteristics of spinal cord reflex reactions in experimental hypoparathyroidism,” Byull. Eksperim. Biol. Med., 86, No. 12, 659–662 (1978).
D. N. Khudaverdyan and V. Z. Grigoryan, “Inhibitory processes in the spinal cord in cats with parathyroprivous tetany,” Dokl. Akad. Nauk SSSR, 246, 753–755 (1979).
D. N. Khudaverdyan, G. G. Artsruni, A. S. Ter-Markosyan, and A. S. Ovsepyan, “Ca2+ contents in liver and brain mitochondria in rats with hypoparathyroidism,” Byull. Eksperim. Biol. Med., XCVII, No. 3, 301–303 (1984).
D. N. Khudaverdyan and A. G. Tatevosyan, “Contents of some neuroactive amino acids in different parts of the brain in experimental hypoparathyroidism,” Zh. Eksperim. Klin. Med. Akad. Nauk. Arm. SSR, 18, No. 2, 19–23 (1987).
D. N. Khudaverdyan and A. S. Ter-Markosyan, “Nervous tissue as a target for the actions of parathyroid hormone,” Usp. Fiziol. Nauk., 23, No. 1, 90–102 (1992).
G. Birke and A. Draguhn, “No simple brake – the complex functions of inhibitory,” Pharmacopsychiatry, 43, No. 1, 21–31 (2010).
N. Burnashev and A. Rozov, “Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy,” Cell Calcium, 37, No. 5, 489–495 (2005).
T. Bouschet and J. M. Henley, “Calcium as an extracellular signaling molecule: perspectives on the Calcium Sensing Receptor in the brain,” C. R. Biol., 328, 691–700 (2005).
R. Cuppini, P. Ambrogini, S. Sartini, et al., “The role of sensory input in motor neuron sprouting control,” Somatosens. Mot. Res., 19, No. 4, 279–285 (2002).
F. A. Davis and C. L. Schauf, “Neural blocking activity of multiple sclerosis and EAE sera,” Neurology, 26, No. 6, part 2, 43–44 (1976).
Z. Fengt and D. M. Durand, “Effects of potassium concentration on firing patterns of low-calcium epileptiform activity in anesthetized rat hippocampus: inducing of persistent spike activity,” Epilepsia, 47, No. 4, 727–736 (2006).
S. Fujii, M. Matsumoto, K. Igarishi, et al., “Synaptic plasticity in hippocampal CA1 neurons of mice lacking type 1 inositol-1,4,5-trisphosphate receptors,” Learn. Mem., 7, 312–320 (2000).
A. A. Galoyan, J. S. Sarkissian, V. A. Chavushyan, et al., “Neuroprotection by hypothalamic peptide proline-rich peptide-1 in Abeta 25-35 model of Alzheimer’s disease,” Alzheim. Dement., 4, No. 5, 332–344 (2008).
A. A. Galoyan, N. Khalaj, L. E. Hambardzumyan, et al., “Protective effects of hypothalamic proline-rich peptide and cobra venom Naja Naja Oxiana on dynamics of vestibular compensation following unilateral labyrinthectomy,” Neurochem. Res., 35, 1747–1760 (2010).
L. Giardino, M. Zanni, M. Fernandez, et al., “Plasticity of GABA(a) system during ageing: focus on vestibular compensation and possible pharmacological intervention,” Brain Res., 929, No. 1, 76–86 (2002).
K. Jensen, M. S. Jensen, and J. D. Lambert, “Role of presynaptic L-type Ca2+ channels in gabaergic synaptic transmission in cultured hippocampal neurons,” J. Neurophysiol., 81, No. 3, 1225–1230 (1999).
A. R. Johnston, A. Him, and M. B. Dutia, “Differential regulation of GABA(A) and GABA(B) receptors during vestibular compensation,” Neuroreport, 12, 597–600 (2001).
D. N. Khudaverdyan and A. S. Ter-Markosyan, “Parathyroid hormone as a modulator of the functional activity of neuron,” Biochemistry (Moscow), 65, No. 2, 171–175 (2000).
M. P. Mattson, “Calcium and neurodegeneration,” Aging Cell., 6, No. 3, 337–350 (2007).
H. Matsui, S. Aou, J. Ma, and T. Hori, “Central actions of parathyroid hormone on blood calcium and hypothalamic neuronal activity in the rat,” Am. J. Physiol., 268, No. 1, part 2, R21–R27 (1995).
R. J. Miller, “Presynaptic receptors,” Annu. Rev. Pharmacol. Toxicol., 38, 201–227 (1998).
D. F. Owens and A. R. Kriegstein, “Is there more to GABA than synaptic inhibition?” Nat. Rev. Neuroscience, 3, No. 9, 715–727 (2002).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Elsevier Academic Press (2005).
J. S. Sarkissian, V. A. Chavushyan, I. Meliksetyan, et al., “Protective effect of Naja naja oxiana cobra venom in rotenone-induced model of Parkinson’s disease: electrophysiological and histochemical analysis,” New Armen. Med. J., 1, 43–56 (2007).
G. E. Stutzmann, A. Caccamo, F. M. LaFerla, and I. Parker, “Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin 1 results in exaggerated Ca2+ signals and altered membrane excitability,” J. Neurosci., 24, 508–513 (2004).
A. S. Ter-Markosyan and D. N. Khudaverdyan, “Cellular mechanism of parathyroid hormone action on the Nerve Tissue,” New Armen. Med. J., 3, No. 2, 6–13 (2009).
B. Tighilet and M. Lacour, “Gamma amino butyric acid (GABA) immunoreactivity in the vestibular nuclei of normal and unilateral vestibular neurectomized cats,” Eur. J. Neurosci., 13, 2255–2267 (2001).
T. Wang, J. Wang, J. E. Cottrell, and I. S. Kass, “Small physiologic changes in calcium and magnesium alter excitability and burst firing of CA1 pyramidal cells in rat hippocampal slices,” J. Neurosurg. Anesthesiol., 16, No. 3, 201–209 (2004).
R. Yuste, A. Majewska, and K. Holthoff, “From form to function: calcium compartmentalization in dendritic spines,” Nat. Neurosci., 3, 653–659 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 100, No. 5, pp. 555–580, May, 2014.
Rights and permissions
About this article
Cite this article
Khudaverdyan, D.N., Avetisyan, K.A., Chavushyan, V.A. et al. Electrophysiological Analysis of the Functional State of Spinal Cord Motoneurons in Rats with Parathyroprivous Tetany. Neurosci Behav Physi 46, 9–27 (2016). https://doi.org/10.1007/s11055-015-0193-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-015-0193-6