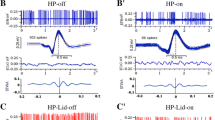
Experiments on 23 white male rats (250 g) analyzed the spike activity of individual neurons in the substantia nigra pars compacta (SNc, 242 neurons, n = 11) and substantia nigra pars reticulata (SNr, 289 neurons, n = 12) during high-frequency stimulation of the primary motor cortex (M1)in normal animals and in animals with a rotenone model of Parkinson’s disease (BP). SNc neurons in the model of PD showed a complete absence of depressor effects induced by stimulation, though tetanic potentiation was accompanied by posttetanic potentiation and depression at levels 1.65 and 2.02 times greater than in normal animals. In SNr neurons in normal animals, tetanic potentiation, accompanied by post-tetanic potentiation and depression, was 2.37 times greater than tetanic depression, while in the model of PD the levels of both depressor and excitatory activity induced by stimulation were below normal. Spike activity frequency in SNc and SNr neurons preceding and accompanying stimulation was significantly greater than normal in the model of PD. This is evidence for excitotoxicity accompanying neurodegenerative damage, which is completed by neuron apoptosis and death. In SNr neurons, both depressor and excitatory reactions accompanying stimulation were markedly dominant over those in SNc neurons, which is evidence for more extensive cortical projections to the SNr. Furthermore, SNc neurons demonstrated greater susceptibility to pathological changes due to poststimulus depressor effects than SNr neurons, with formation of more marked excitatory effects, which is evidence of a greater involvement of the SNc in PD. In the model of PD, lacking stimulation-induced depressor effects and more marked excitatory effects in SNc neurons, SNr neurons retained their depressor reactions and relatively decreased excitatory reactions, which is evidence of a lower level of susceptibility of SNr neurons to excitotoxicity, extreme increases in the excitability of surviving neurons compensating for the lack of excitation of dead cells.
Similar content being viewed by others
References
E. J. Nestler, S. E. Hyman, and R. C. Malenka, Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, McGraw-Hill Medical, New York (2009).
P. Voorn, L. J. Vanderschuren, H. J. Groenewegen, et al., “Putting a spin on the dorsal-ventral divide of the striatum,” Trends Neurosci., 27, 468–474 (2004).
F. M. Zhou and C. R. Lee, “Intrinsic and integrative properties of substantia nigra pars reticulata neurons,” Neuroscience, 198, 69–94 (2011).
E. Guatteo, M. L. Cucchiaroni, and N. B. Mercuri, “Substantia nigra control of basal ganglia nuclei,” J. Neural Transm. (Vienna), Supplement, 73, 91–101 (2009).
J. B. Carman, “Anatomic basis of surgical treatment of Parkinson’s disease,” New Engl. J. Med., 17, 919–930 (1968).
D. R. Weinberger, “Implications of the normal brain development for the pathogenesis of schizophrenia,” Arch. Gen. Psychiatry, 44, 660–669 (1987).
R. A. Wise, “Roles for nigrostriatal – not just mesocorticolimbic – dopamine in reward and addiction,” Trends Neurosci., 32, 517–524 (2009).
E. Düzel, N. Bunzeck, M. Guitart-Masip, et al., “Functional imaging of the human dopaminergic midbrain,” Trends Neurosci., 32, 321–328 (2009).
R. A. Menke, S. Jbabdi, K. L. Miller, et al., “Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson’s disease,” Neuroimage, 52, 1175–1180 (2010).
R. Chowdhury, C. Lambert, R. J. Dolan, and E. Düzel, “Parcellation of the human substantia nigra based on anatomical connectivity to the striatum,” Neuroimage, 81, 191–198 (2013).
B. P. Kolomiets, J. M. Deniau, J. Glowinski, and A. M. Thierry, “Basal ganglia and processing of cortical information: functional interactions between trans-striatal and trans-subthalamic circuits in the substantia nigra pars reticulata,” Neuroscience, 117, No. 4, 931–938 (2003).
H. G. Kwon and S. H. Jang, “Differences in neural connectivity between the substantia nigra and ventral tegmental area in the human brain,” Front. Hum. Neurosci., 8, 41 (2014).
J. Kornhuber, “The cortico-nigral projection: reduced glutamate content in the substantia nigra following frontal cortex ablation in the rat,” Brain Res., 322, 124–126 (1984).
W. G. Frankle, M. Laruelle, and S. N. Haber, “Prefrontal cortical projections to the midbrain in primates: evidence for a sparse connection,” Neuropsychopharmacology, 31, 1627–1636 (2006).
S. R. Sesack and D. B. Carr, “Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia,” Physiol. Behav., 77, 513–517 (2002).
A. Cacciola, D. Milardi, and A. Quartarone, “Role of cortico-pallidal connectivity in the pathophysiology of dystonia,” Brain (2016).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, Elsevier, (2005), 5th ed.
C. Kilkenny, W. J. Browne, I. C. Cuthill, et al., “Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research,” PLoS Biol., 8, No. 6, e1000412 (2010).
W. Schmidt and M. J. Alam, “Controversies on new animal models of Parkinson’s disease pro and con: the rotenone model of Parkinson’s disease (PD),” J. Neural. Transm., 70, Supplement, 273–276 (2006).
M. R. Hynd, H. L. Scott, and P. R. Dodd “Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease,” Neurochem. Int., 45, No. 5, 583–595 (2004).
D. R. Lucas and J. P. Newhouse, “The toxic effect of sodium L-glutamate on the inner layers of the retina,” AMA Arch. Ophthalmol., 58, No. 2, 193–201 (1957).
J. W. Olney, “Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate,” Science, 164, No. 3880, 719–721 (1969).
Xiao-xia Dong, Yan Wang, and Zheng-Hong Qin, “Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases,” Acta Pharmacol. Sin., 30, 379–387 (2009).
J. S. Sarkissian, M. V. Poghosyan, M. A. Danielyan, et al., Purpose of Depressor Synaptic Processes in Conditions of Specific Neurodegenerative Pathology and Protection, LAP LAMBERT Academic Publishing RU (2018).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 106, No. 3, pp. 301–314, March, 2020.
Rights and permissions
About this article
Cite this article
Poghosyan, M.V., Khachatryan, L.M., Danielyan, M.A. et al. Microelectrophysiological Studies of the Ratio of Excitatory to Inhibitory Synaptic Processes in the Corticonigral Projection in a Model of Parkinson’s. Neurosci Behav Physi 50, 1209–1215 (2020). https://doi.org/10.1007/s11055-020-01027-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-01027-5