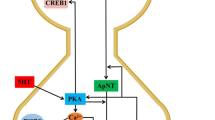
Studies on diaphragm muscle neuromuscular preparations from rats at different states of postnatal development compared the morphological characteristics and functions of the synaptic apparatus, including the time parameters of evoked secretion. Along with nerve ending areas smaller than those in adults, neonates also showed a reduced rate of conduction along motor nerves, reduced intensities of evoked and spontaneous quantum secretion, and very marked fluctuations in true synaptic delays in single-quantum endplate currents. The high level of asynchronicity in the phasic secretion of acetylcholine quanta, along with the longer lifetime of the open state of ion channels in the synapses of neonates, partially compensated for the decrease in the reliability of synaptic transmission due to the reduction in the quantum composition of the postsynaptic response.
Similar content being viewed by others
References
R. J. Balice-Gordon and J. W. Lichtman, “In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions,” J. Neurosci., 73, No. 2, 834–855 (1993).
E. Bukcharaeva, K. Kim, J. Moravec, et al., “Noradrenaline synchronizes quantal release at frog neuromuscular junctions,” J. Physiol., 517, 879–888 (1999).
E. Bukharaeva, D. Samigullin, E. Nikolsky, and L. Magazanik, “Modulation of the kinetics of evoked quantal release at mouse neuromuscular junctions by calcium and strontium,” J. Neurochem., 100, 939–949 (2007).
E. Bukharaeva, D. Samigullin, E. Nikolsky, and F. Vyskocil, “Protein kinase A cascade regulates quantal release dispersion at frog muscle endplate,” J. Physiol., 538, 837–848 (2002).
J. Diamond and R. Miledi, “A study of fetal and newborn rat muscle fibers,” J. Physiol., 162, 393–408 (1962).
M. Favero, G. Busetto, and A. Cangiano, “Spike timing plays a key role in synapse elimination at the neuromuscular junction,” Proc. Natl. Acad. Sci. USA, 109, No. 25, 1667–1675 (2012).
J. D. Feldman, A. R. Bazzy, T. R. Cummins, and G. G. Haddad, “Developmental changes in neuromuscular transmission in the rat diaphragm,” J. Appl. Physiol., 71, 280–286 (1991).
E. Ferraro, F. Molinari, and L. Berghella, “Molecular control of neuromuscular junction development,” J. Cachexia Sarcopenia Muscle, 3, 13–23 (2012).
R. Fesce, “The kinetics of nerve-evoked quantal secretion,” Phil. Trans. Roy. Soc. London, B. Biol. Sci., 354, 319–329 (1999).
M. Fournier, M. Alula, and G. C. Sieck, “Neuromuscular transmission failure during postnatal development,” Neurosci. Lett., 125, 34–36 (1991).
E. Gutmann, V. Hanlikova, and F. Vyskocil, “Age changes in cross-striated muscle of the rat,” J. Physiol., 216, 331–343 (1971).
B. W. Hughes, L. L. Kusner, and H. J. Kaminski, “Molecular architecture of the neuromuscular junction,” Muscle Nerve, 33, 445–461 (2006).
S. Iwasaki, A. Momiyama, O. D. Uchitel, and T. Takahashi, “Developmental changes in calcium channel types mediating central synaptic transmission,” J. Neurosci., 20, No. 1, 59–65 (2000).
B. Katz and R. Miledi, “The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction,” Proc. Roy. Soc. B, 161, 483–495 (1965).
A. M. Kelly and S. I. Zacks, “The fine structure of motor endplate histogenesis,” J. Cell Biol., 42, 154–169 (1969).
S. S. Kelly, “The effect of age on neuromuscular transmission,” J. Physiol., 274, 51–62 (1978).
J.-W. Lin and S. Faber, “Modulation of synaptic delay during synaptic plasticity,” Trends Neurosci., 25, 449–455 (2002).
T. Meier and B. G. Wallace, “Formation of that neuromuscular junction: molecules and mechanisms,” BioEssays, 20, No. 10, 819–829 (1998).
A. R. Punga and M. A. Ruegg, “Signaling and aging at the neuromuscular synapse: lessons learnt from neuromuscular diseases,” Curr. Opin. Pharmacol., 12, 340–346 (2012).
B. Sabatini and W. Regehr, “Timing of synaptic transmission,” Annu. Rev. Physiol., 61, 521–542 (1999).
M. Santafe, A. Garca, M. Lanuza, et al., “Calcium channels coupled to neurotransmitter release at dually innervated neuromuscular junctions in the newborn rat,” Neuroscience, 102, No. 3, 697–708 (2001).
L. Shi, A. K. Fu, and N. Y. Ip, “Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction,” Trends Neurosci., 35, No. 7, 441–453 (2012).
C. Slater, “Reliability of neuromuscular transmission and how it is maintained,” Handb. Clin. Neurol., 91, 27–101 (2008).
B. Soucek, “Influence of latency fluctuations and the quantal process of transmitter release on the end-plate potential’s amplitude distribution,” Biophys. J., 11, 127–139 (1971).
L. Tarsa and Y. Goda, “Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons,” Proc. Natl. Acad. Sci. USA, 99, No. 2, 1012–1016 (2002).
S. G. Turney and J. W. Lichtman, “Reversing the outcome of synapse elimination at developing neuromuscular junctions in vivo: evidence for synaptic competition and its mechanism,” PLoS Biology, 10, No. 6, 1–15 (2012).
A. C. Wareham, R. H. Morton, and G. H. Meakin, “Low quantal content of the endplate potential reduces safety factor for neuromuscular transmission in the diaphragm of the newborn rat,” Brit. J. Anesthesia, 72, 205–209 (1994).
B. Wiedenmann and W. W. Franke, “Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles,” Cell, 41, No. 3, 1017–1028 (1985).
V. Witzemann, “Development of the neuromuscular junction,” Cell. Tiss. Res., 326, 263–271 (2006).
S Wood and C. Slater, “Safety factor at the neuromuscular junction,” Progr. Neurobiol., 64, 393–429 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 98, No. 12, 1544–1554, December, 2012.
Rights and permissions
About this article
Cite this article
Khuzakhmetova, V.F., Samigullin, D.V., Nurullin, L.F. et al. Characteristics of the Transmission of Excitation in Rat Neuromuscular Synapses at Different Periods of Postnatal Development. Neurosci Behav Physi 44, 960–966 (2014). https://doi.org/10.1007/s11055-014-0010-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-014-0010-7