Abstract
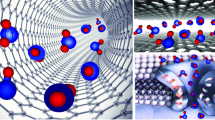
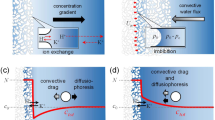
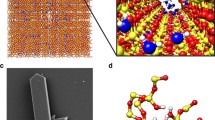
It is of great significance to explore the osmotic transport mechanisms across nanopores for the design and applications of forward osmosis membranes. To deeply understand the quantitative relationship between pore diameters and water transport across nanopores of forward osmosis membrane at molecular level, systematic molecular dynamic simulations are conducted on water transport behaviors across nanopores with a length of 16 Å and with diameters ranging from 8.14 to 16.28 Å. The phase interface barrier on water flux across nanopores is predicted by the potential of mean force and hydrogen-bonding number. The combined effects of water structure, interface barrier and flow resistance of nanopores, and hydrogen bonds on the water flux are analyzed. A non-monotonic profile of the water flux with respect to pore diameters is observed due to the fact that the water flux of forward osmosis is determined by the interface barrier and flow resistance being the same order of magnitude with the naturally osmotic pressure rather than the driven pressure which is one order of magnitude higher than the naturally osmotic pressure. For the pore diameters between 8.14 and 10.85 Å where water structures are highly ordered, the water flux increases with the increasing diameter owing to the combined effects of the increasing diffusion coefficients and decreasing potential of mean force and hydrogen-bonding number. The increasing low resistance within nanopores caused by the water structure transition to be disordered at a critical diameter of 12.21 Å takes account for the obviously decreasing water flux. For the pore diameter being greater than12.21 Å, a linear increase of the water flux with increasing diameter is ascribed to the stabilized diffusion coefficient, potential of mean force, and hydrogen-bonding number.







Similar content being viewed by others
Abbreviations
- D:
-
diffusion coefficient
- HB:
-
hydrogen-bonding
- PMF:
-
potential of mean force
- RDF:
-
radial distribution function
References
Shakeri A, Salehi H, Khankeshipour N et al (2018) Magnetic nanoparticle-crosslinked ferrohydrogel as a novel class of forward osmosis draw agent. J Nanopart Res 20:325. https://doi.org/10.1007/s11051-018-4437-6
Suwaileh W, Pathak N, Shon H et al (2020) Forward osmosis membranes and processes: a comprehensive review of research trends and future outlook. Desalination 485:114455. https://doi.org/10.1016/j.desal.2020.114455
Yip NY, Tiraferri A, Phillip WA et al (2010) High performance thin-film composite forward osmosis membrane. Environ Sci Technol 44(10):3812–3818. https://doi.org/10.1021/es1002555
Arena JT, McCloskey B, Freeman BD et al (2011) Surface modification of thin film composite membrane support layers with polydopamine: enabling use of reverse osmosis membranes in pressure retarded osmosis. J Membr Sci 375:55–62. https://doi.org/10.1016/j.memsci.2011.01.060
Widjojo N, Chung TS, Weber M (2011) The role of sulphonated polymer and macrovoid-free structure in the support layer for thin-film composite (TFC) forward osmosis (FO) membranes. J Membr Sci 383:214–223. https://doi.org/10.1016/j.memsci.2011.08.041
She Q, Wang R, Fane AG, Tang CYY (2016) Membrane fouling in osmotically driven membrane processes: a review. J Membr Sci 499:201–233. https://doi.org/10.1016/j.memsci.2015.10.040
Silva V, Prádanos P, Palacio L, Hernandez A (2009) Alternative pore hindrance factors: what one should be used for nanofiltration modelization? Desalination. 245:606–613. https://doi.org/10.1016/j.desal.2009.02.026
Fierro D, Boschetti-de-Fierro A, Abetz V (2012) The solution-diffusion with imperfections model as a method to understand organic solvent nanofiltration of multicomponent systems. J Membr Sci 413:91–101. https://doi.org/10.1016/j.memsci.2012.04.027
Moradi A, Farsi A, Mansouri SS, Sarcheshmehpoor M (2012) A new approach for modeling of RO membranes using MD-SF-PF model and CFD technique. Res Chem Intermed 38:161–177. https://doi.org/10.1007/s11164-011-0334-7
Matthews TD, Yan H, Cahill DG et al (2013) Growth dynamics of interfacially polymerized polyamide layers by diffuse reflectance spectroscopy and Rutherford backscattering spectrometry. J Membr Sci 429:71–80. https://doi.org/10.1016/j.memsci.2012.11.040
Wang J, Mo Y, Mahendra S (2014) Effects of water chemistry on structure and performance of polyamide composite membranes. J Membr Sci 452:415–425. https://doi.org/10.1016/j.memsci.2013.09.022
Bian LX, Fang YY, Wang XL et al (2016) Analysis of membrane parameters for forward osmosis membrane based on non-equilibrium thermodynamics. Membr Sci Technol 36:75–83. https://doi.org/10.16159/j.cnki.issn1007-8924.2016.04.012
Halder P, Bhattacharya S (2022) Preparations and electrical characterization of copper-molybdate glassy nanocomposites. Nat Conf Condens Matter Phys 66:3381–3386. https://doi.org/10.1016/j.matpr.2022.07.231
Poddar A, Roy M, Bhattacharya S (2022) Dynamics of Ag+ ions in CdI2 doped electrolytes. Nat Conf Condens Matter Phys 66:3373–3376. https://doi.org/10.1016/j.matpr.2022.07.229
Sengupta A, Bhattacharya S, Ghosh CK (2022) Conduction process in some promising lithium-ion doped glassy ceramics. Nat Conf Condens Matter Phys 66:3287–3291. https://doi.org/10.1016/j.matpr.2022.06.410
Yamanaka S, Shimosaka A, Shirakawa Y (2010) Molecular dynamics simulations of the formation for NaCl cluster at the interface between the supersaturated solution and the substrate. J Nanopart Res 12:831–839. https://doi.org/10.1007/s11051-009-9713-z
Ju SP, Lin JS, Hsieh JY et al (2013) Water molecular flow control with a (5,5) nanocoil switch. J Nanopart Res 15:1889. https://doi.org/10.1007/s11051-013-1889-6
Eslami H, Heydari N (2014) Hydrogen bonding in water nano confined between graphene surfaces: a molecular dynamics simulation study. J Nanopart Res 16:2154. https://doi.org/10.1007/s11051-013-2154-8
Gao W, Jiao Y, Dai LL (2016) The effects of size, shape, and surface composition on the diffusive behaviors of nanoparticles at/across water–oil interfaces via molecular dynamics simulations. J Nanopart Res 18:91. https://doi.org/10.1007/s11051-016-3393-2
Sun PF, Yang Z, Song XX et al (2021) Interlayered forward osmosis membranes with MXene and carbon nanotubes for enhanced municipal wastewater concentration. Environ Sci Technol 55:13219–13230. https://doi.org/10.1021/acs.est.1c01968
Liang LJ, Kang ZZ, Shen JW (2016) Translocation mechanism of C-60 and C-60 derivations across a cell membrane. J Nanopart Res 18:333. https://doi.org/10.1007/s11051-016-3647-z
Li L, Zhang YW, Ma HB, Yang M (2010) Molecular dynamics simulation of effect of liquid layering around the nanoparticle on the enhanced thermal conductivity of nanofluids. J Nanopart Res 12:811–821. https://doi.org/10.1007/s11051-009-9728-5
Rissanou AN, Harmandaris V (2013) Structure and dynamics of poly (methylmethacrylate)/graphene systems through atomistic molecular dynamics simulations. J Nanopart Res 15:1589. https://doi.org/10.1007/s11051-013-1589-2
Lu G, Duan YY, Wang XD (2014) Surface tension, viscosity, and rheology of water-based nanofluids: a microscopic interpretation on the molecular level. J Nanopart Res 16:2564. https://doi.org/10.1007/s11051-014-2564-2
Hummer G, Rasaiah JC, Noworyta JP (2001) Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 414:188–190. https://doi.org/10.1038/35102535
Alexiadis A, Kassinos S (2008) Self-diffusivity, hydrogen bonding and density of different water models in carbon nanotubes. Mol Simul 34:671–678. https://doi.org/10.1080/08927020802073057
Zhao KW (2015) Molecular dynamics study on behavior and physical mechanism for water transport across nanochannels. Dissertation. Shanghai Jiaotong University
Zhang X, Zhou W, Xu F et al (2018) Resistance of water transport in carbon nanotube membranes. Nanoscale. 10:13242–13249. https://doi.org/10.1039/c8nr03116a
Varanasi SR, Subramanian Y, Bhatia SK (2018) High interfacial barriers at narrow carbon nanotube–water interfaces. Langmuir. 34:8099–8111. https://doi.org/10.1021/acs.langmuir.8b00616
Suk ME, Aluru NR (2017) Modeling water flow through carbon nanotube membranes with entrance/exit effects. Nanoscale Microscale Thermophys Eng 21:247–262. https://doi.org/10.1080/15567265.2017.1355949
Suk ME, Aluru NR (2010) Water transport through ultrathin graphene. J Phys Chem Lett 1:1590–1594. https://doi.org/10.1021/jz100240r
Ye H, Zhang H, Zheng Y, Zhang ZQ (2011) Nanoconfinement induced anomalous water diffusion inside carbon nanotubes. Microfluid Nanofluidics 10:1359–1364. https://doi.org/10.1007/s10404-011-0772-y
Cohen-Tanugi D, Grossman JC (2012) Water desalination across nanoporous graphene. Nano Lett 12:3602–3608. https://doi.org/10.1021/nl3012853
Zhao KW, Wu HY (2015) Structure-dependent water transport across nanopores of carbon nanotubes: toward selective gating upon temperature regulation. Phys Chem Chem Phys 17:10343–10347. https://doi.org/10.1039/c4cp06054g
Chopra M, Choudhury N (2014) Comparison of structure and dynamics of polar and nonpolar fluids through carbon nanotubes. J Phys Chem C 117:18398–18405. https://doi.org/10.1021/jp404089e
Corry B (2008) Designing carbon nanotube membranes for efficient water desalination. J Phys Chem B 112:1427–1434. https://doi.org/10.1021/jp709845u
Thomas JA, McGaughey AJH (2009) Water flow in carbon nanotubes: transition to subcontinuum transport. Phys Rev Lett 102:184502. https://doi.org/10.1103/PhysRevLett.102.184502
Jia YX, Li Y, Hu YD (2011) Behavior of carbon nanotube membranes as channels of salt and water in forward osmosis process. Acta Phys Chim Sin 27:228–232. https://doi.org/10.3866/PKU.WHXB20110104
Shen C, Guo WL (2019) Manipulation of long-range water ordering in less confined nanotubes. J Phys Chem C 123:10101–10106. https://doi.org/10.1021/acs.jpcc.9b00420
Pan JC, Xiao SB, Zhang ZL et al (2020) Nanoconfined water dynamics in multilayer graphene nanopores. J Phys Chem C 124:17819–17828. https://doi.org/10.1021/acs.jpcc.0c04897
Xu XJ, Zhao YY, Wang JC (2020) Water flow inside various geometric nano-confinement channels. Phys Chem Chem Phys 22:24633–24639. https://doi.org/10.1039/d0cp04491a
Acknowledgements
The authors thank the anonymous reviewers for their constructive and valuable opinions gratefully.
Funding
This work was supported by the National Naturally Science Foundation of China (No. 51976022), the Naturally Science Foundation of Shandong Province of China (No. ZR2021ME074), and the Research Leader’s Studio Project of Universities and Institutes in Jinan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, L., Zhang, Q., Tian, Y. et al. Naturally osmotic water transport across nanopores in relation to pore diameters of forward osmosis membrane. J Nanopart Res 25, 70 (2023). https://doi.org/10.1007/s11051-023-05714-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05714-5