Abstract
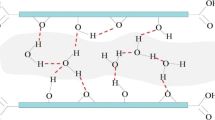
Molecular dynamics simulations are done to study the structure and dynamics of hydrogen bonds (HBs) in water, nanoconfined between parallel graphene surfaces, at a constant parallel component of pressure, 101.3 kPa, and at constant temperatures, ranging from 300 to 390 K. The results indicate that layering of water molecules beside the surfaces strongly influences the structure of HBs. Very close to the surfaces, due to the geometrical restrictions, the hydrogen atoms of water preferentially orient toward the surfaces, and hence, scarify their HB network. The number of HBs per donor, compared to the corresponding bulk value, is reduced in the organized water layers beside the surfaces. In contrary, their number is increased at distances corresponding to the density profile minima, due to the formation of HBs between donors and acceptors in the neighboring organized layers. An analysis of the temperature dependence of the number of HBs shows that the HBs closer to the surfaces are weaker than those in the bulk water. Besides, the entropy change for HB breakage in the pore is lower than that for the bulk water. The short-time behavior of HB dynamics, with a characteristic time ≈0.1 ps, is shown to be insensitive to the surface proximity. However, an examination of the long-time relaxation of HBs indicates that HBs closer to the surface relax slower than those in the bulk. Such a slower relaxation is shown to be connected to the slower diffusive motion of water, depending on the surface proximity. Consequently, the rate constants for forward (HB breakage) and backward (HB formation) reactions in the pore are smaller than the corresponding bulk values. In all cases, the effect of surfaces is shown to extend up to ≈1.0 nm (interphase thickness) from the surfaces.









Similar content being viewed by others
References
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Berendsen HJC, Grigera JR, Straatsma TP (1987) The missing term in effective pair potentials. J Phys Chem 91:6269–6271
Choudhury N, Pettitt BM (2005) On the mechanism of hydrophobic association of nanoscopic solutes. J Am Chem Soc 127:3556–3567
Cicero G, Grossman JC, Schwegler E, Gygi F, Galli G (2008) Water confined in nanotubes and between graphene sheets: a first principle study. J Am Chem Soc 130:1871–1878
Eslami H, Mehdipour N (2012) Local chemical potential and pressure tensor in inhomogeneous nanoconfined fluids. J Chem Phys 137:144702
Eslami H, Müller-Plathe F (2009) Structure and mobility of nanoconfined polyamide-6,6 oligomers: application of a molecular dynamics technique with constant temperature, surface area, and parallel pressure. J Phys Chem B 113:5568–5581
Eslami H, Müller-Plathe F (2010) Viscosity of nanoconfined polyamide-6,6 oligomers: atomistic reverse nonequilibrium molecular dynamics simulation. J Phys Chem B 114:387–395
Eslami H, Müller-Plathe F (2013) How thick is the interphase in an ultrathin polymer film? coarse-grained molecular dynamics simulations of polyamide-6,6 on graphene. J Phys Chem C 117:5249–5257
Eslami H, Mozaffari F, Moghadasi J, Müller-Plathe F (2008) Molecular dynamics simulation of confined fluids in isosurface-isothermal-isobaric ensemble. J Chem Phys 129:194702
Eslami H, Mohammadzadeh L, Mehdipour N (2012) Anisotropic heat transport in nanoconfined polyamide-6,6 oligomers: atomistic reverse nonequilibrium molecular dynamics simulation. J Chem Phys 136:104901
Eslami H, Jaafari B, Mehdipour N (2013) Coarse grained molecular dynamics simulation of nanoconfined water. ChemPhysChem 14:1063–1070
Hirunsit P, Balbuena PB (2007) Effects of confinement on water structure and dynamics: a molecular simulation study. J Phys Chem C 111:1709–1715
Huang DM, Chandler D (2000) Temperature and length scale dependence of hydrophobic effects and their possible implications for protein folding. Proc Natl Acad Sci USA 97:8324–8327
Huang DM, Chandler D (2002) The hydrophobic effect and the influence of solute–solvent attractions. J Phys Chem B 106:2047–2053
Koga K, Gao GT, Tanaka H, Zeng XC (2002) How does water freeze inside carbon nanotubes? Phys A 314:462–469
Kolesnikov AI, Zanotti JM, Loong C-K, Thiyagarajan P, Moravsky AP, Loutfy RO, Burham CJ (2004) Anomalously soft dynamics of water in a nanotube: a revelation of nanoscale confinement. Phys Rev Lett 93:035503
Luna M, Colchero J, Baro AM (1999) Study of water droplets and films on graphite by noncontact scanning force microscopy. J Phys Chem B 103:9576–9581
Luzar A (2000) Resolving the hydrogen bond dynamics conundrum. J Chem Phys 113:10663
Luzar A, Chandler D (1996) Hydrogen-bond kinetics in liquid water. Nature (London) 379:55–58
Maibaum L, Chandler D (2003) A coarse-grained model of water confined in a hydrophobic tube. J Phys Chem B 107:1189–1193
Marti J, Nagy G, Gordillo MC, Guardia E (2006) Molecular simulation of liquid water confined inside graphite channels: thermodynamics and structural properties. J Chem Phys 124:09470
Milischuk AA, Ladanyi BM (2011) Structure and dynamics of water confined in silica nanopores. J Chem Phys 135:174709
Müller-Plathe F (1993) YASP: a molecular simulation package. Comput Phys Commun 78:77–94
Perkyns JS, Pettitt BM (1996) Dependence of hydration free energy on solute size. J Phys Chem 100:1323–1329
Rapaport DC (1983) Hydrogen bonds in water. Mol Phys 50:1151–1162
Schwendel D, Hayashi T, Dahint R, Pertsin A, Grunze M, Steitz R, Schreiber F (2003) Interaction of water with self-assembled monolayers: neutron reflectivity measurements of the water density in the interface region. Langmuir 19:2284–2293
Wallqvist A, Berne BJ (1995) Computer simulation of hydrophobic hydration forces on stacked plates at short range. J Phys Chem 99:2893–2899
Yaminsky VV, Amelina EA, Shchukin ED (1983) Interaction between particles in a nonwetting liquid. Colloids Surf 6:68–75
Zangi R, Mark AE (2003) Bilayer ice and alternate liquid phases of confined water. J Chem Phys 119:1694
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eslami, H., Heydari, N. Hydrogen bonding in water nanoconfined between graphene surfaces: a molecular dynamics simulation study. J Nanopart Res 16, 2154 (2014). https://doi.org/10.1007/s11051-013-2154-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-2154-8