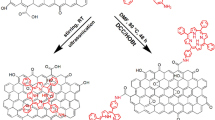
Abstract
Graphene-porphyrin hybrid materials with direct assembly between the fluorinated graphene (FG) nanosheets and the pre-formed 5,10,15,20-tetrakis(p-hydroxyphenyl)porphyrin (THPP) units have been successfully synthesized. The structure–property, binding, and assembly of THPP over FG nanosheets were methodically analyzed by spectroscopic, microscopic, and solvent wetting techniques. The solvent wetting characteristics and surface free energy of the supportless THPP and FG-supported THPP were investigated. The decoration of porphyrin over FG ensued in a surface free energy of 28.7 mJ m−2 directing to hydrophobic (WCA ~ 130 ± 2°) networks. Photo-physical studies demonstrated considerable non-covalent interactions between the THPP with FG multi-layers. The emission responses of THPP and FG-THPP with ferric complexes and its coordination with respect to the –philicity of the THPP were probed using photoluminescence spectroscopy. The functional groups blended in the FG-THPP inks were subjected to fuse with interconnected networks like cellulose paper and melamine sponge by exploiting the dispersion processability followed by morphology evaluation and water-wettability.
Graphical abstract










Similar content being viewed by others
References
Ariga K (2021) Nanoarchitectonics with porphyrins and related molecules. J Porphyr Phthalocyanines 25(10n12):897–916. https://doi.org/10.1142/s1088424621300056
Auwärter W, Écija D, Klappenberger F, Barth JV (2015) Porphyrins at interfaces Nat Chem 7(2):105–120. https://doi.org/10.1038/nchem.2159
Lu H, Kobayashi N (2016) Optically active porphyrin and phthalocyanine systems. Chem Rev 116(10):6184–6261. https://doi.org/10.1021/acs.chemrev.5b00588
Senge MO, Sergeeva NN, Hale KJ (2021) Classic highlights in porphyrin and porphyrinoid total synthesis and biosynthesis. Chem Soc Rev 50(7):4730–4789. https://doi.org/10.1039/C7CS00719A
Ishihara S, Labuta J, Van Rossom W, Ishikawa D, Minami K, Hill JP, Ariga K (2014) Porphyrin-based sensor nanoarchitectonics in diverse physical detection modes. Phys Chem Chem Phys 16(21):9713–9746. https://doi.org/10.1039/C3CP55431G
Lim JM, Yoon ZS, Shin J-Y, Kim KS, Yoon M-C, Kim D (2008) The photophysical properties of expanded porphyrins: relationships between aromaticity, molecular geometry and non-linear optical properties. Chem Commun 3:273. https://doi.org/10.1039/B810718A
Bizaia N, de Faria EH, Ricci GP, Calefi PS, Nassar EJ, Castro KADF, Nakagaki S, Ciuffi KJ, Trujillano R, Vicente MA, Gil A, Korili SA (2009) Porphyrin−kaolinite as efficient catalyst for oxidation reactions. ACS Appl Mater Interfaces 1(11):2667–2678. https://doi.org/10.1021/am900556b
Canton-Vitoria R, Scharl T, Stergiou A, Cadranel A, Arenal R, Guldi DM, Tagmatarchis N (2020) Ping-pong energy transfer in covalently linked porphyrin-MoS2 architectures. Angew Chem Int Ed 59(10):3976–3981. https://doi.org/10.1002/anie.201914494
Chen J, Zhu Y, Kaskel S (2021) Porphyrin-based metal–organic frameworks for biomedical applications. Angew Chem Int Ed 60(10):5010–5035. https://doi.org/10.1002/anie.201909880
Chen R, Wang Y, Ma Y, Mal A, Gao X-Y, Gao L, Qiao L, Li X-B, Wu L-Z, Wang C (2021) Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution. Nat Commun 12(1):1354. https://doi.org/10.1038/s41467-021-21527-3
Jin L, Lv S, Miao Y, Liu D, Song F (2021) Recent development of porous porphyrin-based nanomaterials for photocatalysis. ChemCatChem 13(1):140–152. https://doi.org/10.1002/cctc.202001179
Liang Z, Wang H-Y, Zheng H, Zhang W, Cao R (2021) Porphyrin-based frameworks for oxygen electrocatalysis and catalytic reduction of carbon dioxide. Chem Soc Rev 50(4):2540–2581. https://doi.org/10.1039/D0CS01482F
Silvestri S, Fajardo AR, Iglesias BA (2022) Supported porphyrins for the photocatalytic degradation of organic contaminants in water: a review. Environ Chem Lett 20(1):731–771. https://doi.org/10.1007/s10311-021-01344-2
Wang C, Hao Y, Wang Y, Song H, Hussain S, Gao R, Gao L, He Y, Zheng G, Tang Y (2021) Multiwall carbon nanotubes non-covalently functionalized by porphyrin–Sn networks for protein adsorption. ACS Appl Nano Mater 4(3):2345–2350. https://doi.org/10.1021/acsanm.0c03215
Wei Y, Zhang M, Wu P, Luo J, Dai L, Li H, Wang L, She Y, Zhu W, Li H-m (2020) Tailoring electronic properties of porphyrin manganese on boron nitride for enhancing aerobic oxidative desulfurization at room temperature. ACS Sustain Chem Eng 8(2):1015–1022. https://doi.org/10.1021/acssuschemeng.9b05728
Zhang N, Wang L, Wang H, Cao R, Wang J, Bai F, Fan H (2018) Self-assembled one-dimensional porphyrin nanostructures with enhanced photocatalytic hydrogen generation. Nano Lett 18(1):560–566. https://doi.org/10.1021/acs.nanolett.7b04701
Fujimura T, Shimada T, Sasai R, Takagi S (2018) Optical humidity sensing using transparent hybrid film composed of cationic magnesium porphyrin and clay mineral. Langmuir 34(12):3572–3577. https://doi.org/10.1021/acs.langmuir.7b04006
Monteiro AR, Neves MGPMS, Trindade T (2020) Functionalization of graphene oxide with porphyrins: synthetic routes and biological applications. ChemPlusChem 85(8):1857–1880. https://doi.org/10.1002/cplu.202000455
Zhang B, Gu Q, Wang C, Gao Q, Guo J, Wong PW, Liu CT, An AK (2021) Self-assembled hydrophobic/hydrophilic porphyrin-Ti3C2Tx MXene Janus membrane for dual-functional enabled photothermal desalination. ACS Appl Mater Interfaces 13(3):3762–3770. https://doi.org/10.1021/acsami.0c16054
Bera R, Mandal S, Mondal B, Jana B, Nayak SK, Patra A (2016) Graphene–porphyrin nanorod composites for solar light harvesting. ACS Sustain Chem 4(3):1562–1568. https://doi.org/10.1021/acssuschemeng.5b01504
Dasler D, Schäfer RA, Minameyer MB, Hitzenberger JF, Hauke F, Drewello T, Hirsch A (2017) Direct covalent coupling of porphyrins to graphene. J Am Chem Soc 139(34):11760–11765. https://doi.org/10.1021/jacs.7b04122
Du Y, Dong N, Zhang M, Zhu K, Na R, Zhang S, Sun N, Wang G, Wang J (2017) Covalent functionalization of graphene oxide with porphyrin and porphyrin incorporated polymers for optical limiting. Phys Chem Chem Phys 19(3):2252–2260. https://doi.org/10.1039/C6CP05920A
Gacka E, Burdzinski G, Marciniak B, Kubas A, Lewandowska-Andralojc A (2020) Interaction of light with a non-covalent zinc porphyrin–graphene oxide nanohybrid. Phys Chem Chem Phys 22(24):13456–13466. https://doi.org/10.1039/D0CP02545C
Gacka E, Wojcik A, Mazurkiewicz-Pawlicka M, Malolepszy A, Stobiński L, Kubas A, Hug GL, Marciniak B, Lewandowska-Andralojc A (2019) Noncovalent porphyrin–graphene oxide nanohybrids: the pH-dependent behavior. J Phys Chem C 123(6):3368–3380. https://doi.org/10.1021/acs.jpcc.8b11374
Karousis N, Sandanayaka ASD, Hasobe T, Economopoulos SP, Sarantopoulou E, Tagmatarchis N (2011) Graphene oxide with covalently linked porphyrin antennae: synthesis, characterization and photophysical properties. J Mater Chem 21(1):109–117. https://doi.org/10.1039/C0JM00991A
Mondal B, Bera R, Nayak SK, Patra A (2016) Graphene induced porphyrin nano-aggregates for efficient electron transfer and photocurrent generation. J Mater Chem C 4(25):6027–6036. https://doi.org/10.1039/C6TC01580H
Wang A, Song J, Jia D, Yu W, Long L, Song Y, Cifuentes MP, Humphrey MG, Zhang L, Shao J, Zhang C (2016) Functionalization of reduced graphene oxide with axially-coordinated metal-porphyrins: facile syntheses and temporally-dependent nonlinear optical properties. Inorg Chem Front 3(2):296–305. https://doi.org/10.1039/C5QI00209E
Xu Y, Liu Z, Zhang X, Wang Y, Tian J, Huang Y, Ma Y, Zhang X, Chen Y (2009) A graphene hybrid material covalently functionalized with porphyrin: synthesis and optical limiting property. Adv Mater 21(12):1275–1279. https://doi.org/10.1002/adma.200801617
Yamuna R, Ramakrishnan S, Dhara K, Devi R, Kothurkar NK, Kirubha E, Palanisamy PK (2013) Synthesis, characterization, and nonlinear optical properties of graphene oxide functionalized with tetra-amino porphyrin. J Nanoparticle Res 15(1):1399. https://doi.org/10.1007/s11051-012-1399-y
Datta KKR, Zboril R (2014) Halogenated graphenes: emerging family of two-dimensional materials. Wiley-VCH Verlag GmbH & Co. KGaA, Functionalization of Graphene. https://doi.org/10.1002/9783527672790.ch6
Karlický F, Kumara Ramanatha Datta K, Otyepka M, Zbořil R (2013) Halogenated graphenes: rapidly growing family of graphene derivatives. ACS Nano 7(8):6434–6464. https://doi.org/10.1021/nn4024027
Chen X, Fan K, Liu Y, Li Y, Liu X, Feng W, Wang X (2022) Recent advances in fluorinated graphene from synthesis to applications: critical review on functional chemistry and structure engineering. Adv Mater 34(1):2101665. https://doi.org/10.1002/adma.202101665
Matochová D, Medved M, Bakandritsos A, Steklý T, Zbořil R, Otyepka M (2018) 2D Chemistry: chemical control of graphene derivatization. J Phys Chem Lett 9(13):3580–3585. https://doi.org/10.1021/acs.jpclett.8b01596
Borse RA, Kale MB, Sonawane SH, Wang Y (2022) Fluorographene and its composites: fundamentals, electrophysical properties, DFT studies, and advanced applications. Adv Funct Mater 32(26):2202570. https://doi.org/10.1002/adfm.202202570
Luo Z, Wang X, Chen D, Chang Q, Xie S, Ma Z, Lei W, Pan J, Pan Y, Huang J (2021) Ultrafast Li/fluorinated graphene primary batteries with high energy density and power density. ACS Appl Mater Interfaces 13(16):18809–18820. https://doi.org/10.1021/acsami.1c02064
Prasanthi I, Raidongia K, Datta KKR (2021) Super-wetting properties of functionalized fluorinated graphene and its application in oil–water and emulsion separation. Mater Chem Front 5(16):6244–6255. https://doi.org/10.1039/d1qm00757b
Ravi Y, Prasanthi I, Behera S, Datta KKR (2022) MIL-101(Fe) networks supported on fluorinated graphene nanosheets as coatings for oil sorption. ACS Appl Nano Mater 5(4):5857–5867. https://doi.org/10.1021/acsanm.2c01083
Šedajová V, Bakandritsos A, Błoński P, Medveď M, Langer R, Zaoralová D, Ugolotti J, Dzíbelová J, Jakubec P, Kupka V, Otyepka M (2022) Nitrogen doped graphene with diamond-like bonds achieves unprecedented energy density at high power in a symmetric sustainable supercapacitor. Energy Environ Sci 15(2):740–748. https://doi.org/10.1039/D1EE02234B
Ye X, Ma L, Yang Z, Wang J, Wang H, Yang S (2016) Covalent functionalization of fluorinated graphene and subsequent application as water-based lubricant additive. ACS Appl Mater Interfaces 8(11):7483–7488. https://doi.org/10.1021/acsami.5b10579
Yogapriya R, Datta KKR (2020) Porous fluorinated graphene and ZIF-67 composites with hydrophobic-oleophilic properties towards oil and organic solvent sorption. J Nanosci Nanotechnol 20(5):2930–2938. https://doi.org/10.1166/jnn.2020.17465
Yogapriya R, Kasibhatta KRD (2020) Hydrophobic-superoleophilic fluorinated graphene nanosheet composites with metal–organic framework HKUST-1 for oil–water separation. ACS Appl Nano Mater 3(6):5816–5825. https://doi.org/10.1021/acsanm.0c00980
Zoppellaro G, Bakandritsos A, Tuček J, Błoński P, Susi T, Lazar P, Bad’ura Z, Steklý T, Opletalová A, Otyepka M, Zbořil R (2019) Microwave energy drives “On–Off–On” spin-switch behavior in nitrogen-doped graphene. Adv Mater 31(37):1902587. https://doi.org/10.1002/adma.201902587
Jeon K-J, Lee Z, Pollak E, Moreschini L, Bostwick A, Park C-M, Mendelsberg R, Radmilovic V, Kostecki R, Richardson TJ, Rotenberg E (2011) Fluorographene: a wide bandgap semiconductor with ultraviolet luminescence. ACS Nano 5(2):1042–1046. https://doi.org/10.1021/nn1025274
Okotrub AV, Chekhova GN, Pinakov DV, Yushina IV, Bulusheva LG (2022) Optical absorption and photoluminescence of partially fluorinated graphite crystallites. Carbon 193:98–106. https://doi.org/10.1016/j.carbon.2022.03.034
Papadakis I, Kyrginas D, Stathis A, Couris S, Potsi G, Bourlinos AB, Tomanec O, Otyepka M, Zboril R (2019) Large enhancement of the nonlinear optical response of fluorographene by chemical functionalization: the case of diethyl-amino-fluorographene. J Phys Chem C 123(42):25856–25862. https://doi.org/10.1021/acs.jpcc.9b07493
Stathis A, Papadakis I, Karampitsos N, Couris S, Potsi G, Bourlinos AB, Otyepka M, Zboril R (2019) Thiophenol-modified fluorographene derivatives for nonlinear optical applications. ChemPlusChem 84(9):1288–1298. https://doi.org/10.1002/cplu.201800643
Stathis A, Stavrou M, Papadakis I, Bakandritsos A, Steklý T, Otyepka M, Couris S (2020) Octylamine-modified fluorographenes as a versatile platform for the efficient engineering of the nonlinear optical properties of fluorinated graphenes. Adv photonics 1(2):2000014. https://doi.org/10.1002/adpr.202000014
Ariga K (2021) Nanoarchitectonics revolution and evolution: from small science to big technology. Small Science 1(1):2000032. https://doi.org/10.1002/smsc.202000032
Ariga K (2021) Nanoarchitectonics: what’s coming next after nanotechnology? Nanoscale Horiz 6(5):364–378. https://doi.org/10.1039/D0NH00680G
Chaikittisilp W, Yamauchi Y, Ariga K (2022) Material evolution with nanotechnology, nanoarchitectonics, and materials informatics: what will be the next paradigm shift in nanoporous materials? Adv Mater 34(7):2107212. https://doi.org/10.1002/adma.202107212
Chen Y, Huang Z-H, Yue M, Kang F (2014) Integrating porphyrin nanoparticles into a 2D graphene matrix for free-standing nanohybrid films with enhanced visible-light photocatalytic activity. Nanoscale 6(2):978–985. https://doi.org/10.1039/C3NR04908F
Jing J, Yang J, Li W, Wu Z, Zhu Y (2022) Construction of interfacial electric field via dual-porphyrin heterostructure boosting photocatalytic hydrogen evolution. Adv Mater 34(3):2106807. https://doi.org/10.1002/adma.202106807
Gkini K, Verykios A, Balis N, Kaltzoglou A, Papadakis M, Adamis KS, Armadorou K-K, Soultati A, Drivas C, Gardelis S, Petsalakis ID, Palilis LC, Fakharuddin A, Haider MI, Bao X, Kennou S, Argitis P, Schmidt-Mende L, Coutsolelos AG, Falaras P, Vasilopoulou M (2020) Enhanced organic and perovskite solar cell performance through modification of the electron-selective contact with a bodipy–porphyrin dyad. ACS Appl Mater Interfaces 12(1):1120–1131. https://doi.org/10.1021/acsami.9b17580
Min KS, Manivannan R, Son Y-A (2019) Porphyrin Dye/TiO2 imbedded PET to improve visible-light photocatalytic activity and organosilicon attachment to enrich hydrophobicity to attain an efficient self-cleaning material. Dyes Pigm 162:8–17. https://doi.org/10.1016/j.dyepig.2018.10.014
Reddy G, Katakam R, Devulapally K, Jones LA, Della Gaspera E, Upadhyaya HM, Islavath N, Giribabu L (2019) Ambient stable, hydrophobic, electrically conductive porphyrin hole-extracting materials for printable perovskite solar cells. J Mater Chem C 7(16):4702–4708. https://doi.org/10.1039/C9TC00605B
Kitazaki Y, Hata T (1972) Surface-chemical criteria for optimum adhesion. J Adhes 4(2):123–132. https://doi.org/10.1080/00218467208072217
Law K-Y, Zhao H (2016) Determination of solid surface tension by contact angle. In: Law K-Y, Zhao H (eds) Surface wetting: characterization, contact angle, and fundamentals. Springer International Publishing, Cham, pp 135–148. https://doi.org/10.1007/978-3-319-25214-8_7
Larowska D, Wojcik A, Mazurkiewicz-Pawlicka M, Malolepszy A, Stobiński L, Marciniak B, Lewandowska-Andralojc A (2019) Cationic porphyrin-graphene oxide hybrid: donor-acceptor composite for efficient photoinduced electron transfer. ChemPhysChem 20(8):1054–1066. https://doi.org/10.1002/cphc.201900040
Qi Z-L, Cheng Y-H, Xu Z, Chen M-L (2020) Recent advances in porphyrin-based materials for metal ions detection. Int J Mol Sci 21(16):5839. https://doi.org/10.3390/ijms21165839
Acknowledgements
Authors acknowledge DST-SERB (ECR/2017/002075) for the financial assistance to this project and thank DST-FIST Department of Chemistry for SR/FST/CST-266/2015(c) and MNRE for the TEM facility. S.B. acknowledges DST-INSPIRE Fellowship [No. DST/INSPIRE Fellowship/2021/IF200405]. The central facilities at SRM IST are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Behera, S., Datta, K.K.R. Rational design of fluorinated graphene-porphyrin nanoarchitectonics: integrating hydrophobicity to macromolecular heterocyclic systems. J Nanopart Res 24, 175 (2022). https://doi.org/10.1007/s11051-022-05556-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05556-7