Abstract
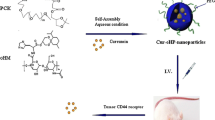
A series of novel hydroxycamptothecin (HCPT) conjugates (13a–14d), which contained a polyethylene glycol moiety and disulfide bond, were designed and synthesized in five to six steps, with overall yields of 20–39%. The anticancer activities and toxicities of these new conjugates were evaluated using an in vitro MTT assay in K562, HepG2, and HT-29 cell lines and HUVECs. The conjugates displayed enhanced antitumor activity and reduced toxicity in comparison with their parent molecule, HCPT. Among these conjugates, compound 13a exhibited 100-fold better selectivity to the tumor cells than to HUVECs. TEM and DLS experiments demonstrated that 13a formed nanosized micelles with a diameter of approximately 200 nm in aqueous solution and that the conjugate could undergo glutathione-responsive degradation to release HCPT at the tumor site. The improved potency and reduced toxicity of these conjugates may be caused by the enhanced permeation and retention (EPR) effect of nanoparticles.








Similar content being viewed by others
References
Blanco E, Hsiao A, Ruiz-Esparza GU, Landry MG, Meric-Bernstam F, Ferrari M (2011) Molecular-targeted nanotherapies in cancer: enabling treatment specificity. Mol Oncol 5:492–503. doi:10.1016/j.molonc.2011.10.005
Cheetham AG, Zhang P, Lin YA, Lock LL, Cui H (2013) Supramolecular nanostructures formed by anticancer drug assembly. J Am Chem Soc 135:2907–2910. doi:10.1021/ja3115983
Chen W, Shi Y, Feng H, Du M, Zhang JZ, Hu J, Yang D (2012) Preparation of copolymer paclitaxel covalently linked via a disulfide bond and its application on controlled drug delivery. J Phys Chem B 116:9231–9237. doi:10.1021/jp303260f
Denison TA, Bae YH (2012) Tumor heterogeneity and its implication for drug delivery. J Control Release 164:187–191. doi:10.1016/j.jconrel.2012.04.014
Gao Y et al (2011) Controlled intracellular release of doxorubicin in multidrug-resistant cancer cells by tuning the shell-pore sizes of mesoporous silica nanoparticles. ACS Nano 5:9788–9798. doi:10.1021/nn2033105
Jackson AW, Fulton DA (2012) Triggering polymeric nanoparticle disassembly through the simultaneous. Application of Two Different Stimuli Macromolecules 45:2699–2708
Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC (2012) Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev 41:2971–3010. doi:10.1039/c2cs15344k
Li XQ et al (2011) Self-assembling nanomicelles of a novel camptothecin prodrug engineered with a redox-responsive release mechanism. Chem Commun (Camb) 47:8647–8649. doi:10.1039/c1cc12495a
Lu J et al (2015) The self-assembling camptothecin-tocopherol prodrug: an effective approach for formulating camptothecin. Biomaterials 62:176–187. doi:10.1016/j.biomaterials.2015.05.046
Maeda H, Matsumura Y (2011) EPR effect based drug design and clinical outlook for enhanced cancer chemotherapy. Adv Drug Deliv Rev 63:129–130. doi:10.1016/j.addr.2010.05.001
Matsumura Y (2011) Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv Drug Deliv Rev 63:184–192. doi:10.1016/j.addr.2010.05.008
Mullen DG, Banaszak Holl MM (2011) Heterogeneous ligand-nanoparticle distributions: a major obstacle to scientific understanding and commercial translation. Acc Chem Res 44:1135–1145. doi:10.1021/ar1001389
Nalwa HS (2011) Encyclopedia of nanoscience and nanotechnology, 15-volume set (Vols. 11–25) 2011.
Ryu JH, Chacko RT, Jiwpanich S, Bickerton S, Babu RP, Thayumanavan S (2010) Self-cross-linked polymer nanogels: a versatile nanoscopic drug delivery platform. J Am Chem Soc 132:17227–17235. doi:10.1021/ja1069932
Tai W, Mo R, Lu Y, Jiang T, Gu Z (2014) Folding graft copolymer with pendant drug segments for co-delivery of anticancer drugs. Biomaterials 35:7194–7203. doi:10.1016/j.biomaterials.2014.05.004
Veronese FM, Pasut G (2005) PEGylation, successful approach to drug delivery. Drug Discov Today 10:1451–1458. doi:10.1016/S1359-6446(05)03575-0
Wang J et al (2013) Tumor redox heterogeneity-responsive prodrug nanocapsules for cancer chemotherapy. Adv Mater 25:3670–3676. doi:10.1002/adma.201300929
Wei H, Schellinger JG, Chu DS, Pun SH (2012) Neuron-targeted copolymers with sheddable shielding blocks synthesized using a reducible, RAFT-ATRP double-head agent. J Am Chem Soc 134:16554–16557. doi:10.1021/ja3085803
Xing T, Mao C, Lai B, Yan L (2012) Synthesis of disulfide-cross-linked polypeptide nanogel conjugated with a near-infrared fluorescence probe for direct imaging of reduction-induced drug release. ACS Appl Mater Interfaces 4:5662–5672. doi:10.1021/am301600u
Xu Z, Wang D, Xu S, Liu X, Zhang X, Zhang H (2014) Preparation of a camptothecin prodrug with glutathione-responsive disulfide linker for anticancer drug delivery. Chem Asian J 9:199–205. doi:10.1002/asia.201301030
Zheng ZB, Zhu G, Tak H, Joseph E, Eiseman JL, Creighton DJ (2005) N-(2-hydroxypropyl)methacrylamide copolymers of a glutathione (GSH)-activated glyoxalase i inhibitor and DNA alkylating agent: synthesis, reaction kinetics with GSH, and in vitro antitumor activities. Bioconjug Chem 16:598–607. doi:10.1021/bc0499634
Zhou L et al (2011) Endosomal pH-activatable poly(ethylene oxide)-graft-doxorubicin prodrugs: synthesis, drug release, and biodistribution in tumor-bearing mice. Biomacromolecules 12:1460–1467. doi:10.1021/bm101340u
Acknowledgements
The authors are thankful to the Research Centre of Modern Analytical Technology, Tianjin University of Science and Technology, for NMR measurements and MALDI-TOF analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81302649) and Natural Science Foundation of Tianjin City (14JCQNJC13200 and 16JCTPJC46000).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Guo, N., Hao, T., Shang, X. et al. Novel amphiphilic PEG-hydroxycamptothecin conjugates as glutathione-responsive prodrug nanocapsules for cancer chemotherapy. J Nanopart Res 19, 205 (2017). https://doi.org/10.1007/s11051-017-3897-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3897-4