Abstract
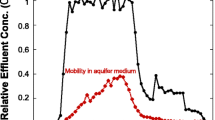
Carbon-based engineered nanoparticles have been widely used due to their small size and unique physical and chemical properties. At the same time, the toxic effects of these nanoparticles on human and fish cells have also been observed; therefore, their release and distribution into the surface and subsurface environment is a subject of concern. The aim of this research is to evaluate and compare the transports and retentions of two types of engineered nanoparticles (multiwalled carbon nanotubes and C60) and the natural carbon nanoparticles collected from a fire accident. Several laboratory experiments were conducted to observe the transport behavior of nanoparticles through a column packed with silica sand. The column experiments were intended to monitor the effect of ionic strength on transport of nanoparticles as a function of their shapes. It was observed that the mobilities of both types of engineered nanoparticles were reduced with the increasing ionic strength from 1.34 to 60 mM. However, at ionic strengths up to 10.89 mM, spherical nanoparticles were more mobile than cylindrical nanoparticles, but the mobility of the cylindrical nanoparticles became significantly higher than spherical nanoparticles at the ionic strength of 60 mM. In comparison with natural fire-born nanoparticles, both types of engineered nanoparticles were much less mobile under the selected experimental condition in this study. Furthermore, inverse modeling was used to calculate parameters such as attachment efficiency, the longitudinal dispersivity, and capacity of the solid phase for the attachment of nanoparticles. The results indicate that the combination of the shape and the solution chemistry of the NPs are responsible for the transport and the retention of nanoparticles in natural environment; however, fire-burned nanoparticles can be highly mobile at the natural groundwater chemistry.






Similar content being viewed by others
References
Arbuckle WB, Ho YF (1990) Adsorber column diameter: particle diameter ratio requirements. Res J Water Pollut Control Fed 62:88–90
Bhattacharjee S, Elimelech M (1997) Surface element integration: a novel technique for evaluation of DLVO interaction between a particle and a flat plate. J Colloid Interface Sci 193:273–285
Bradford SA, Yates SR, Bettahar M, Simunek J (2002) Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resour Res 38:1327. doi:10.1029/2002WR001340
Brant JA, Lecoanet H, Wiesner MR (2005) Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanopart Res 7:545–553
Brant JA, Labille J, Bottero J-Y, Wiesner MR (2006) Characterizing the impact of preparation method on fullerene cluster structure and chemistry. Langmuir 22:3878–3885
Chen KL, Elimelech M (2006) Aggregation and deposition kinetics of fullerene (C60) nanoparticles. Langmuir 22:10994–11001
Grassian VH (2008) Nanoscience and nanotechnology environmental and health impacts. Wiley, New York
Gregory J (1981) Approximate expressions for retarded van der waals interaction. J Colloid Interface Sci 83:138–145
He H, Pham-Huy LA, Dramou P, Xiao D, Zuo P, Pham-Huy C (2013) Carbon nanotubes: applications in pharmacy and medicine. Biomed Res Int 2013:e578290. doi:10.1155/2013/578290
Hertzberg T, Blomqvist P (2003) Particles from fire—a screening of common materials found in buildings. Fire Mater 27:295–314
Hotze EM, Phenrat T, Lowry GV (2010) Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual 39:1909–1924
Hyung H, Fortner JD, Hughes JB, Kim J-H (2007) Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ Sci Technol 41:179–184
Jaisi DP, Saleh NB, Blake RE, Elimelech M (2008) Transport of single-walled carbon nanotubes in porous media: filtration mechanisms and reversibility. Environ Sci Technol 42:8317–8323
Jiang J, Oberdörster G, Biswas P (2009) Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res 11:77–89
Kasel D, Bradford SA, Simunek J, Heggen M, Vereecken H, Klumpp E (2013) Transport and retention of multi-walled carbon nanotubes in saturated porous media: effects of input concentration and grain size. Water Res 47:913–944
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem SETAC 27:1825–1851
Lind CJ (1970) Specific conductance as a means of estimating ionic strength. U.S. Geological Survey Professional Paper 700-D, p D272–D280
Liu J, Rinzler A, Dai H, Hafner J, Bradley R, Boul P, Lu A, Iverson T, Shelimov K, Huffman C, Rodriguez-Macias F, Shon Y, Lee T, Colbert D, Smalley R (1998) Fullerene pipes. Science 280:1253–1256
Liu X, O’Carroll DM, Petersen EJ, Huang Q, Anderson CL (2009) Mobility of multiwalled carbon nanotubes in porous media. Environ Sci Technol 43:8153–8158
Mashayekhi H, Ghosh S, Du P, Xing B (2012) Effect of natural organic matter on aggregation behavior of C60 fullerene in water. J Colloid Interface Sci 374:111–117
Mattison NT, O’Carroll DM, Kerry Rowe R, Petersen EJ (2011) Impact of porous media grain size on the transport of multi-walled carbon nanotubes. Environ Sci Technol 45:9765–9775
Mekonen A, Sharma P, Fagerlund F (2014) Transport and mobilization of multiwall carbon nanotubes in quartz sand under varying saturation. Environ Earth Sci 71:3751–3760
Montgomery SW, Franchek MA, Goldschmidt VW (2000) Analytical dispersion force calculations for nontraditional geometries. J Colloid Interface Sci 227:567–584
Nisbet ICT, LaGoy PK (1992) Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs). Regul Toxicol Pharmacol 16:290–300
O’Carroll DM, Liu X, Mattison NT, Petersen EJ (2013) Impact of diameter on carbon nanotube transport in sand. J Colloid Interface Sci 390:96–104
Petersen EJ, Zhang L, Mattison NT, O’Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang Q, Henry TB, Holbrook RD, Chen KL (2011) Potential release pathways, environmental fate, and ecological risks of carbon nanotubes. Environ Sci Technol 45:9837–9856
Seymour MB, Chen G, Su C, Li Y (2013) Transport and retention of colloids in porous media: Does shape really matter? Environ Sci Technol 47:8391–8398
Sharma P (2012) Geological disposal of nuclear waste: fate and transport of radioactive materials. In: Ahmed W (ed) Nuclear power—practical aspects. InTech, Rijeka
Sharma P, Fagerlund F (2015) Transport of surface-modified carbon nanotubes through a soil column. J Vis Exp. doi:10.3791/52634
Sharma P, Abdou H, Flury M (2008a) Effect of the lower boundary condition and flotation on colloid mobilization in unsaturated sandy sediments. Vadose Zone J 7:930–940
Sharma P, Flury M, Zhou J (2008b) Detachment of colloids from a solid surface by a moving air–water interface. J Colloid Interface Sci 326:143–150
Sharma P, Flury M, Mattson ED (2008c) Studying colloid transport in porous media using a geocentrifuge. Water Resour Res 44:W07407. doi:10.1029/2007WR006456
Sharma P, Bao D, Fagerlund F (2014) Deposition and mobilization of functionalized multiwall carbon nanotubes in saturated porous media: effect of grain size, flow velocity and solution chemistry. Environ Earth Sci 72:3025–3035
Sharma P, Fagerlund F, Iverfeldt U, Eskebaek A (2016) Fate and transport of fire-born particles in porous media. Technologies 4:2. doi:10.3390/technologies4010002
Tan CW, Tan KH, Ong YT, Mohamed AR, Zein SHS, Tan SH (2012) Energy and environmental applications of carbon nanotubes. Environ Chem Lett 10:265–273
Tian Y, Gao B, Wu L, Munoz R, Huang Q (2012) Effect of solution chemistry on multi-walled carbon nanotube deposition and mobilization in clean porous media. J Hazard Mater 231–232:79–87
Vold MJ (1954) Van der Waals’ attraction between anisometric particles. J Colloid Sci 9:451–459
Wang Y, Li Y, Pennell KD (2008) Influence of electrolyte species and concentration on the aggregation and transport of fullerene nanoparticles in quartz sands. Environ Toxicol Chem SETAC 27:1860–1867
Wang J, Chen Y, Blau WJ (2009) Carbon nanotubes and nanotube composites for nonlinear optical devices. J Mater Chem 19:7425–7443. doi:10.1039/B906294G
Xu M, Eckstein Y (1997) Statistical analysis of the relationships between dispersivity and other physical properties of porous media. Hydrogeol J 5:4–20
Yang Y, Nakada N, Nakajima R, Yasojima M, Wang C, Tanaka H (2013) pH, ionic strength and dissolved organic matter alter aggregation of fullerene C60 nanoparticles suspensions in wastewater. J Hazard Mater 244–245:582–587
Zhang L, Hou L, Wang L, Kan AT, Chen W, Tomson MB (2012) Transport of fullerene nanoparticles (nC60) in saturated sand and sandy soil: controlling factors and modeling. Environ Sci Technol 46:7230–7238
Zhang L, Zhang Y, Lin X, Yang K, Lin D (2014) The role of humic acid in stabilizing fullerene (C60) suspensions. J Zhejiang Univ Sci 15:634–642
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedayati, M., Sharma, P., Katyal, D. et al. Transport and retention of carbon-based engineered and natural nanoparticles through saturated porous media. J Nanopart Res 18, 57 (2016). https://doi.org/10.1007/s11051-016-3365-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3365-6