Abstract
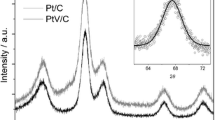
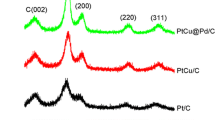
In order to clarify the effect of reduction enhancer on the nanoparticle formation process and their structural and catalytic properties, carbon-supported Pt–Cu nanoparticles were synthesized by electron beam irradiation on an aqueous precursor solution in the presence/absence of reduction enhancer. In the absence of reduction enhancer, tetravalent platinum oxide particles of approximately 1 nm in diameter were formed on carbon support with copper barely precipitated, while in the presence of 2-propanol or ethylene glycol or glucose both platinum and copper precipitated as few-nanometer-sized alloy particles together with copper oxides. It was suggested that the metal nuclei produced upon electron beam irradiation do not have enough lifetime without reduction enhancer due to fast oxidation of the nuclei by oxidizing radicals, while the reduction enhancer scavenges these oxidizing radicals preventing oxidation of metallic clusters and prolonging their lifetime. Ethylene glycol gave smaller and better alloyed particles with less copper oxides compared to 2-propanol since the carbonyl compounds derived from oxidation of ethylene glycol protect metallic clusters from oxidation further prolonging their lifetime. In the electrochemical measurements, the methanol oxidation activities of Pt–Cu/C catalysts were well explained by their structural characteristics.










Similar content being viewed by others
References
Anniyev T, Ogasawara H, Ljungberg MP, Wikfeldt KT, MacNaughton JB, Naslund LA, Bergmann U, Koh S, Strasser P, Pettersson LGM, Nilsson A (2010) Complementarity between high-energy photoelectron and L-edge spectroscopy for probing the electronic structure of 5d transition metal catalysts. Phys Chem Chem Phys 12:5694–5700. doi:10.1039/b926414k
Bai L, Zhu H, Thrasher JS, Street SC (2009) Synthesis and electrocatalytic activity of photoreduced platinum nanoparticles in a poly(ethylenimine) matrix. ACS Appl Mater Interfaces 1:2304–2311. doi:10.1021/am900471f
Barr NF, Allen AO (1959) Hydrogen atoms in the radiolysis of water. J Phys Chem 63:928–931. doi:10.1021/j150576a037
Barta J, Pospisil M, Cuba V (2010) Photo- and radiation-induced preparation of nanocrystalline copper and cuprous oxide catalysts. J Radioanal Nucl Chem 286:611–618. doi:10.1007/s10967-010-0748-5
Belloni J (2006) Nucleation, growth and properties of nanoclusters studied by radiation chemistry—application to catalysis. Catal Today 113:141–156. doi:10.1016/j.cattod.2005.11.082
Belloni J, Mostafavi M (2001) Radiation chemistry of nanocolloids and clusters. In: Jonah CD, Rao BSM (eds) Radiation chemistry: present status and future trends, studies in physical and theoretical chemistry, vol 87. Elsevier Science, Amsterdam, pp 411–452
Belloni J, Mostafavi M, Remita H, Marignier JL, Delcourt MO (1998) Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J Chem 22:1239–1255. doi:10.1039/A801445K
Bergamaski K, Pinheiro ALN, Teixeira-Neto E, Nart FC (2006) Nanoparticle size effects on methanol electrochemical oxidation on carbon supported platinum catalysts. J Phys Chem B 110:19271–19279. doi:10.1021/jp063337h
Bock C, Paquet C, Couillard M, Botton GA, MacDougall BR (2004) Size-selected synthesis of PtRu nano-catalysts: reaction and size control mechanism. J Am Chem Soc 126:8028–8037. doi:10.1021/ja0495819
Chen W, Kim J, Sun S, Chen S (2007) Composition effects of FePt alloy nanoparticles on the electro-oxidation of formic acid. Langmuir 23:11303–11310. doi:10.1021/la7016648
Corradini PG, Pires FI, Paganin VA, Perez J, Antolini E (2012) Effect of the relationship between particle size, inter-particle distance, and metal loading of carbon supported fuel cell catalysts on their catalytic activity. J Nanopart Res 14:1080. doi:10.1007/s11051-012-1080-5
Daimon H, Kurobe Y (2006) Size reduction of PtRu catalyst particle deposited on carbon support by addition of non-metallic elements. Catal Today 111:182–187. doi:10.1016/j.cattod.2005.10.023
Daimon H, Kitakami O, Fujiwara H (1989) A new method for controlling coercivity of iron deposited alumite films. J Magn Soc Jpn 13:795–800. doi:10.3379/jmsjmag.13.S1_795
Daimon H, Onodera T, Honda Y, Nitani H, Seino S, Nakagawa T, Yamamoto TA (2008) Activity and durability of PtRuP catalysts and their atomic structures. ECS Trans 11:93–100. doi:10.1149/1.2992497
Dixon D, Melke J, Botros M, Rathore J, Ehrenberg H, Roth C (2013) Increase of catalyst utilization in polymer electrolyte membrane fuel cells by shape-selected Pt nanoparticles. Int J Hydrogen Energy 38:13393–13398. doi:10.1016/j.ijhydene.2013.07.110
Ferrin P, Mavrikakis M (2009) Structure sensitivity of methanol electrooxidation on transition metals. J Am Chem Soc 131:14381–14389. doi:10.1021/ja904010u
Guo JW, Zhao TS, Prabhuram J, Wong CW (2005) Preparation and the physical/electrochemical properties of a Pt/C nanocatalyst stabilized by citric acid for polymer electrolyte fuel cells. Electrochim Acta 50:1973–1983. doi:10.1016/j.electacta.2004.09.006
Heggen M, Oezaslan M, Houben L, Strasser P (2012) Formation and analysis of core-shell fine structures in Pt bimetallic nanoparticle fuel cell electrocatalysts. J Phys Chem C 116:19073–19083. doi:10.1021/jp306426a
Hsieh CT, Lin JY (2009) Fabrication of bimetallic Pt-M (M = Fe, Co, and Ni) nanoparticle/carbon nanotube electrocatalysts for direct methanol fuel cells. J Power Sources 188:347–352. doi:10.1016/j.jpowsour.2008.12.031
Jayasayee K, Van Veen JAR, Manivasagam TG, Celebi S, Hensen EJM, de Bruijn FA (2012) Oxygen reduction reaction (ORR) activity and durability of carbon supported PtM (Co, Ni, Cu) alloys: influence of particle size and non-noble metals. Appl Catal B 111:515–526. doi:10.1016/j.apcatb.2011.11.003
Kageyama S, Seino S, Nakagawa T, Nitani H, Ueno K, Daimon H, Yamamoto TA (2011) Formation of PtRu alloy nanoparticle catalyst by radiolytic process assisted by addition of dl-tartaric acid and its enhanced methanol oxidation activity. J Nanopart Res 13:5275–5287. doi:10.1007/s11051-011-0513-x
Kinoshita K, Stonehart P (1975) Role of platinum surface morphology on hydrogen adsorption isotherms-IV. Electrochim Acta 20:101–107. doi:10.1016/0013-4686(75)85050-X
Kinoshita K, Lundquist J, Stonehart P (1973) Hydrogen adsorption on high surface area platinum crystallites. J Catal 31:325–334. doi:10.1016/0021-9517(73)90302-3
Koh S, Strasser P (2007) Electrocatalysis on bimetallic surfaces: modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J Am Chem Soc 129:12624–12625. doi:10.1021/ja0742784
Kugai J, Kitagawa R, Seino S, Nakagawa T, Ohkubo Y, Nitani H, Daimon H, Yamamoto TA (2011) γ-Fe2O3-supported Pt-Cu nanoparticles synthesized by radiolytic process for catalytic CO preferential oxidation. Appl Catal A 406:43–50. doi:10.1016/j.apcata.2011.08.006
Kuo PL, Chen WF, Huang HY, Chang IC, Dai SA (2006) Stabilizing effect of pseudo-dendritic polyethylenimine on platinum nanoparticles supported on carbon. J Phys Chem B 110:3071–3077. doi:10.1021/jp055688m
Lei M, Liang C, Huan Q, Miyabayashi K, Miyake M, Yang T (2014) Morphology-controlled growth of Pt nanoparticles taking advantage of smaller molecule and inorganic salt. Acta Mater 63:202–208. doi:10.1016/j.actamat.2013.10.028
Ozkar S, Finke RG (2002) Nanocluster formation and stabilization fundamental studies: ranking commonly employed anionic stabilizers via the development, then application, of five comparative criteria. J Am Chem Soc 124:5796–5810. doi:10.1021/ja012749v
Papadimitriou S et al (2010) Methanol oxidation at Pt-Cu, Pt-Ni, and Pt-Co electrode coatings prepared by a galvanic replacement process. J Phys Chem C 114:5217–5223. doi:10.1021/jp911568g
Peng X, Zhao Y, Chen D, Fan Y, Wang X, Wang W, Tian J (2014) One-pot synthesis of reduced graphene oxide supported PtCuy catalysts with enhanced electro-catalytic activity for the methanol oxidation reaction. Electrochim Acta 136:292–300. doi:10.1016/j.electacta.2014.05.110
Rao CS, Singh DM, Sekhar R, Rangarajan J (2011) Pt-Co electrocatalyst with varying atomic percentage of transition metal. Int J Hydrogen Energy 36:14805–14814. doi:10.1016/j.ijhydene.2010.12.142
Ross PN Jr (1979) Structure sensitivity in electrocatalytic properties of Pt: II. Oxygen reduction on low index single crystals and the role of steps. J Electrochem Soc 126:78–82. doi:10.1149/1.2128991
Roy PS, Bhattacharya SK (2013) Size-controlled synthesis and characterization of polyvinyl alcohol-coated platinum nanoparticles: role of particle size and capping polymer on the electrocatalytic activity. Catal Sci Technol 3:1314–1323. doi:10.1039/C3CY20686F
Seino S, Kinoshita T, Nakagawa T, Kojima T, Taniguci R, Okuda S, Yamamoto TA (2008) Radiation induced synthesis of gold/iron-oxide composite nanoparticles using high-energy electron beam. J Nanopart Res 10:1071–1076. doi:10.1007/s11051-007-9334-3
Soroushian B, Lampre I, Belloni J, Mostafavi M (2005) Radiolysis of silver ion solutions in ethylene glycol: solvated electron and radical scavenging yields. Radiat Phys Chem 72:111–118. doi:10.1016/j.radphyschem.2004.02.009
Stamenkovic V, Mun BS, Mayrhofer KJJ, Ross PN, Markovic NM, Rossmeisl J, Greeley J, Norskov JK (2006a) Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew Chem Int Ed Engl 45:2897–2901. doi:10.1002/anie.200504386
Stamenkovic VR, Mun BS, Mayrhofer KJJ, Ross PN, Markovic NM (2006b) Effect of surface composition on electronic structure, stability, and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces. J Am Chem Soc 128:8813–8819. doi:10.1021/ja0600476
Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C, Liu Z, Kaya S, Nordlund D, Ogasawara H, Toney MF, Nilsson A (2010) Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat Chem 2:454–460. doi:10.1038/nchem.623
Takasu Y, Iwazaki T, Sugimoto W, Murakami Y (2000) Size effects of platinum particles on the electro-oxidation of methanol in an aqueous solution of HClO4. Electrochem Commun 2:671–674. doi:10.1016/S1388-2481(00)00101-6
Vegard L (1921) The constitution of the mixed crystals and the filling of space of the atoms. Z Phys 5:17–26. doi:10.1007/BF01349680
Wang H, Wang R, Li H, Wang Q, Kang J, Lei Z (2011) Facile synthesis of carbon-supported pseudo-core@shell PdCu@Pt nanoparticles for direct methanol fuel cells. Int J Hydrogen Energy 36:839–848. doi:10.1016/j.ijhydene.2010.09.033
Xu Y, Xie X, Guo J, Wang S, Wang Y, Mathur VK (2006) Effects of annealing treatment and pH on preparation of citrate-stabilized PtRu/C catalyst. J Power Sources 162:132–140. doi:10.1016/j.jpowsour.2006.07.021
Xu C, Liu Y, Wang J, Geng H, Qiu H (2011) Fabrication of nanoporous Cu-Pt(Pd) core/shell structure by galvanic replacement and its application in electrocatalysis. Appl Mater Interfaces 3:4626–4632. doi:10.1021/am201057t
Yahikozawa K, Fujii Y, Matsuda Y, Nishimura K, Takasu Y (1991) Electrocatalytic properties of ultrafine platinum particles for oxidation of methanol and formic acid in aqueous solutions. Electrochim Acta 36:973–978. doi:10.1016/0013-4686(91)85302-N
Yamamoto TA, Nakagawa T, Seino S, Nitani H (2010) Bimetallic nanoparticles of PtM (M = Au, Cu, Ni) supported on iron oxide: radiolytic synthesis and CO oxidation catalysis. Appl Catal A 387:195–202. doi:10.1016/j.apcata.2010.08.020
Yamamoto TA, Kageyama S, Seino S, Nitani H, Nakagawa T, Horioka R, Honda Y, Ueno K, Daimon H (2011) Methanol oxidation catalysis and substructure of PtRu/C bimetallic nanoparticles synthesized by a radiolytic process. Appl Catal A 396:68–75. doi:10.1016/j.apcata.2011.01.037
Acknowledgments
The authors thank Mr. K. Ueno (EBIS, Japan) for the provision of beam time for the electron accelerator. The authors thank partial supports from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid No. 22241023 and No. 25410201), and Ministry of Economy, Trade and Industry (R&D Project for Regional Innovation No. 22U5009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kugai, J., Kubota, C., Okazaki, T. et al. Effect of reduction enhancer on a radiolytic synthesis of carbon-supported Pt–Cu nanoparticles and their structural and electrochemical properties. J Nanopart Res 17, 239 (2015). https://doi.org/10.1007/s11051-015-3048-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3048-8