Abstract
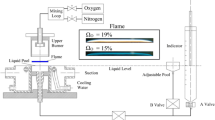
This paper investigates the influence of flame parameters including oxygen concentration, fuel composition, and strain rate on the synthesis of carbon nanomaterials in opposed-jet ethylene diffusion flames with or without rigid-body rotation. In the experiments, a mixture of ethylene and nitrogen was introduced from the upper burner; meanwhile, a mixture of oxygen and nitrogen was supplied from the lower burner. A nascent nickel mesh was used as the catalytic metal substrate to collect deposited materials. With non-rotating opposed-jet diffusion flames, carbon nanotubes (CNTs) were successfully produced for oxygen concentrations in the range of 21–50 % at a fixed ethylene concentration of 20 %, and for ethylene concentrations ranging from 14 to 24 % at a constant oxygen concentration of 40 %. With rotating opposed-jet diffusion flames, the strain rate was varied by adjusting the angular velocities of the upper and lower burners. The strain rate governed by flow rotation greatly affects the synthesis of carbon nanomaterials [i.e., CNTs and carbon nano-onions (CNOs)] either through the residence time or carbon sources available. An increase in the angular velocity lengthened the residence time of the flow and thus caused the diffusion flame to experience a decreased strain rate, which in turn produced more carbon sources. The growth of multi-walled CNTs was achieved for the stretched flames experiencing a higher strain rate [i.e., angular velocity was equal to 0 or 1 rotations per second (rps)]. CNOs were synthesized at a lower strain rate (i.e., angular velocity was in the range of 2–5 rps). It is noteworthy that the strain rate controlled by flow rotation greatly influences the fabrication of carbon nanostructures owing to the residence time as well as carbon source. Additionally, more carbon sources and higher temperature are required for the synthesis of CNOs compared with those required for CNTs (i.e., about 605–625 °C for CNTs and 700–800 °C for CNOs).







Similar content being viewed by others
References
Andrews R, Jacques D, Rao AM, Derbyshire F, Qian D, Fan X, Dickey EC (1999) Continuous production of aligned carbon nanotubes: a step closer to commercial realization. Chem Phys Lett 303:467–474
Borgohain R, Yang J, Selegue JP, Kim DY (2014) Controlled synthesis, efficient purification, and electrochemical characterization of arc-discharge carbon nano-onions. Carbon 66:272–284
Choucair M, Stride JA (2012) The gram-scale synthesis of carbon onions. Carbon 50:1109–1115
Chung DH, Lin TH, Hou SS (2010) Flame synthesis of carbon nano-onions enhanced by acoustic modulation. Nanotechnology 21:435604
de Heer WA, Ugarte D (1993) Carbon onions produced by heat treatment of carbon soot and their relation to the 217.5 nm interstellar absorption feature. Chem Phys Lett 207:480–486
Dhand V, Prasad JS, Rao MV, Bharadwaj S, Anjaneyulu Y, Jain PK (2013) Flame synthesis of carbon nano onions using liquefied petroleum gas without catalyst. Mater Sci Eng C 33:758–762
Guo T, Nikolaev P, Thess A, Colbert DT, Smalley RE (1995) Catalytic growth of single-walled manotubes by laser vaporization. Chem Phys Lett 243:49–54
Height MJ, Howard JB, Tester JW (2005) Flame synthesis of single walled carbon nanotubes. Proc Combust Inst 30:2537–2543
Hou SS, Chang KC, Lin TH (1992) Analysis of finite laminar opposed-jets with and without rigid-body rotation. Int J Heat Mass Transf 35:945–956
Hou SS, Yang SS, Chen SJ, Lin TH (2003) Interactions for flames in a coaxial flow with a stagnation point flow. Combust Flame 132:58–72
Hou SS, Chung DH, Lin TH (2009a) Flame synthesis of carbon nanotubes in a rotating counterflow. J Nanosci Nanotechnol 9:4826–4833
Hou SS, Chung DH, Lin TH (2009b) High-yield synthesis of carbon nano-onions in counterflow diffusion flames. Carbon 47:938–947
Hou SS, Huang WC, Lin TH (2012) Flame synthesis of carbon nano-structures using mixed fuel in oxygen-enriched environment. J Nanopart Res 14:1243
Hu WC, Hou SS, Lin TH (2014) Analysis on controlling factors for the synthesis of carbon nanotubes and nano-onions in counterflow diffusion flames. J Nanosci Nanotechnol 14:5363–5369
Huang WC, Hou SS (2013) Influence of flow rotation on synthesis of carbon nano-structures via diffusion flame method. In: National Conference on Combustion Science and Technology. Hsinchu, Taiwan
Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE (1985) C60: Buckminsterfullerene. Nature 318:162–163
Lee GW, Jurng J, Hwang J (2004) Synthesis of carbon nanotubes on a catalytic metal substrate by using an ethylene inverse diffusion flame. Carbon 42:682–685
Li TX, Zhang HG, Wang FJ, Chen Z, Saito K (2007) Synthesis of carbon nanotubes on Ni-alloy and Si-substrates using counterflow methane–air diffusion flames. Proc Combust Inst 31:1849–1856
Li TX, Kuwana K, Saito K, Zhang H, Chen Z (2009) Temperature and carbon source effects on methane–air flame synthesis of CNTs. Proc Combust Inst 32:1855–1861
Lin TH, Sohrab SH (1987) Influence of vorticity on counterflow diffusion flames. Combust Sci Technol 52:75–90
Lin TH, Sohrab SH (1989) Diffusion flames in opposed counter-rotating jet. Combust Sci Technol 63:193–207
Merchan-Merchan W, Saveliev AV, Kennedy LA, Fridman A (2002) Formation of carbon nanotubes in counter-flow, oxy-methane diffusion flames without catalyst. Chem Phys Lett 354:20–24
Merchan-Merchan W, Saveliev AV, Kennedy LA (2004) High-rate flame synthesis of vertically aligned carbon nanotubes using electric field control. Carbon 42:599–608
Merchan-Merchan W, Saveliev A, Kennedy LA, Jimenez C (2010) Combustion synthesis of carbon nanotubes and related nanostructure. Prog Energy Combust Sci 36:696–727
Morjan IP, Alexandrescu R, Morjan I, Luculescu C, Vasile E, Osiceanu P, Scarisoreanu M, Demian G (2013) Effect of the manufacturing parameters on the structure of nitrogen-doped carbon nanotubes produced by catalytic laser-induced chemical vapor deposition. J Nanopart Res 15:2045
Nakazawa S, Yokomori T, Mizomoto M (2005) Flame synthesis of carbon nanotubes in a wall stagnation flow. Chem Phys Lett 403:158–162
Rua C, Lian Y (2015) Purification of carbon nano-onions fabricated by arc discharge. Fuller Nanotub Carbon N 23:488–493
Saveliev AV, Kennedy LA, Merchan-Merchan W (2003) Metal catalyzed synthesis of carbon nanostructures in an opposed flow methane oxygen flame. Combust Flame 135:27–33
Seymour MB, Su C, Gao Y, Lu Y, Li Y (2012) Characterization of carbon nano-onions for heavy metal ion remediation. J Nanopart Res 15:2045
Tsuji H (1982) Counterflow diffusion flames. Prog Energy Combust Sci 8:93–119
Ugarte D (1992) Curling and closure of graphitic networks under electron-beam irradiation. Nature 359:707–709
Unrau CJ, Axelbaum RL, Fraundorf P (2010) Single-walled carbon nanotube formation on iron oxide catalysts in diffusion flames. J Nanopart Res 12:2125–2133
Vander Wal RL, Ticich TM (2001) Comparative flame and furnace synthesis of single-walled carbon nanotubes. Chem Phys Lett 336:24–32
Vander Wal RL, Hall LJ, Berger GMT (2002) The chemistry of premixed flame synthesis of carbon nanotubes using supported catalysts. Proc Combust Inst 29:1079–1085
Wang X, Hu Y, Liu C, Long Y, Xu S, Zhu D, Dai L (2001) Bamboo-like carbon nanotubes produced by pyrolysis of iron(II) phthalocyanine. Carbon 39:1533–1536
Wang Q, Sun X, He D, Zhang J (2013) Preparation and study of carbon nano-onion for lithium storage. Mater Chem Phys 139:333–337
Xu F, Liu X, Tse SD (2006) Synthesis of carbon nanotubes on metal alloy substrates with voltage bias in methane diffusion flames. Carbon 44:570–577
Xu F, Zhao H, Tse SD (2007) Carbon nanotube synthesis on catalytic metal alloys in methane/air counterflow diffusion flames. Proc Combust Inst 31:1839–1847
Yuan L, Saito K, Hu W, Chen Z (2001a) Ethylene flame synthesis of well aligned multi-walled carbon nanotubes. Chem Phys Lett 346:23–28
Yuan L, Saito K, Pan C, Williams FA, Gordon AS (2001b) Nanotubes from methane flames. Chem Phys Lett 340:237–241
Yuan L, Li TX, Saito K (2003) Growth mechanism of carbon nanotubes in methane diffusion flames. Carbon 41:1889–1996
Zhang W, Fu J, Chang J, Zhang M, Yang Y, Gao L (2015) Fabrication and purification of carbon nano onions. Carbon 82:610
Acknowledgments
The authors would like to thank the National Science Council, Taiwan, ROC, for financially supporting this research under Grant NSC 100-2221-E-168-036-MY2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, SS., Huang, WC. Influence of oxygen concentration, fuel composition, and strain rate on synthesis of carbon nanomaterials. J Nanopart Res 17, 65 (2015). https://doi.org/10.1007/s11051-015-2879-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-2879-7