Abstract
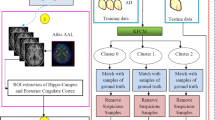
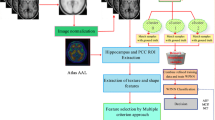
A progressive brain disorder, which eventually destroys memory cells, is termed Alzheimer’s Disease (AD). AD causes memory loss and other regular activities. Due to the variations in cytoarchitecture, the categorical labeling of various tissues presents a difficult task in AD classification. For addressing this challenge, this paper proposes a new GELU and SWISH-based Radial Basis Function Network (GS-RBFN)-centric early prediction and classification of AD. For classifying AD into Mild Cognitive Impairment (MCI), AD, and Control Normal (CN), the proposed model deploys image pre-processing, segmentation, morphological operation, data augmentation, image representation extraction, feature selection, and classification steps. Primarily, images are gathered from the Alzheimer's Disease Neuroimaging Initiative (ADNI) dataset. Next, by utilizing normalization, skull removal, and spatial smoothing approaches, the images are pre-processed. Then, by using the Brownian Log Scaling Archimedes Optimization-based Watershed Segmentation (BLSAOWS), significant brain tissues are segmented. After that, using morphological operations, the segmented images are enhanced. Next, for obtaining different formations of the segmented images, a data augmentation process is deployed. Subsequently, the image features are extracted, and the best features are chosen utilizing the Base Switch Rule Infimum and Supremum-centric Rock Hyrax Swarm Optimization (BSRISRHSO) algorithm. Lastly, utilizing a new GS-RBFN classifier, the AD is classified. Through the experimental analysis, the proposed model’s efficiency is determined. Thus, the proposed GS-RBFN proficiently predicts AD individuals with an accuracy, precision, and sensitivity of 98.45%, 98.44%, and 98.44%, respectively. The proposed GS-RBFN achieved a less computation time of 14876 ms. Furthermore, the proposed BSRISRHSO obtained a minimum feature selection time of 24012 ms. The Proposed BLSAOWS acquired a high efficiency of 98%. Also, the proposed model acquired superior accuracy that outperformed all baseline techniques. Thus, the experimental results revealed that the research methodology obtained more impressive outcomes in AD prediction.








Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Lei B, Yang M, Yang P, Zhou F, Hou W, Zou W, Li X, Wang T, Xiao X, Wang S (2020) Deep and joint learning of longitudinal data for Alzheimer’s disease prediction. Pattern Recogn 102:1–25. https://doi.org/10.1016/j.patcog.2020.107247
Hussain E, Hasan M, Hassan SZ, Azmi TH, Rahman MA, Parvez MZ (2020) Deep learning based binary classification for alzheimer’s disease detection using brain MRI Images. In: 2020 15th IEEE Conference on Industrial Electronics and Applications (ICIEA). IEEE, Kristiansand, pp 1115–1120. https://doi.org/10.1109/ICIEA48937.2020.9248213
Dua M, Makhija D, Manasa PYL, Mishra P (2020) A CNN–RNN–LSTM Based amalgamation for alzheimer’s disease detection. J Med Biol Eng 40(5):688–706. https://doi.org/10.1007/s40846-020-00556-1
Neelaveni J, Devasana MSG (2020) Alzheimer disease prediction using machine learning algorithms. In: 2020 6th International Conference on Advanced Computing and Communication Systems (ICACCS). IEEE, Coimbatore, pp 101–104. https://doi.org/10.1109/ICACCS48705.2020.9074248
Tabarestani S, Aghili M, Eslami M, Cabrerizo M, Barreto A, Rishe N, Curiel RE, Loewenstein D, Duara R, Adjouadi M (2020) A distributed multitask multimodal approach for the prediction of Alzheimer’s disease in a longitudinal study. Neuroimage 206:1–15. https://doi.org/10.1016/j.neuroimage.2019.116317
Salehi AW, Baglat P, Sharma BB, Gupta G, Upadhya A (2020) A CNN model: earlier diagnosis and classification of alzheimer disease using MRI. In: 2020 International Conference on Smart Electronics and Communication (ICOSEC). IEEE, Trichy, pp 156–161. https://doi.org/10.1109/ICOSEC49089.2020.9215402
Bi X, Li S, Xiao B, Li Y, Wang G, Ma X (2020) Computer aided Alzheimer’s disease diagnosis by an unsupervised deep learning technology. Neurocomputing 392(2019):296–304. https://doi.org/10.1016/j.neucom.2018.11.111
Ljubic B, Roychoudhury S, Cao XH, Pavlovski M, Obradovic S, Nair R, Glass L, Obradovic Z (2020) Influence of medical domain knowledge on deep learning for Alzheimer’s disease prediction. Comput Methods Programs Biomed 197:1–7. https://doi.org/10.1016/j.cmpb.2020.105765
Jung W, Jun E, Suk HI (2021) Deep recurrent model for individualized prediction of Alzheimer’s disease progression. Neuroimage 237:1–20. https://doi.org/10.1016/j.neuroimage.2021.118143
Liu J, Li M, Luo Y, Yang S, Li W, Bi Y (2021) Alzheimer’s disease detection using depthwise separable convolutional neural networks. Comput Methods Programs Biomed 203:1–10. https://doi.org/10.1016/j.cmpb.2021.106032
Ebrahimi A, Luo S, Chiong R (2021) Deep sequence modelling for Alzheimer’s disease detection using MRI. Comput Biol Med 134:1–13. https://doi.org/10.1016/j.compbiomed.2021.104537
Abed MT, Fatema U, Nabil SA, Alam MA, Reza MT (2020) Alzheimer’s disease prediction using convolutional neural network models leveraging pre-existing architecture and transfer learning. In: 2020 Joint 9th International Conference on Informatics, Electronics & Vision (ICIEV) and 2020 4th International Conference on Imaging, Vision & Pattern Recognition (icIVPR). IEEE, Kitakyushu, pp 1–6. https://doi.org/10.1109/ICIEVicIVPR48672.2020.9306649
Bron EE, Klein S, Papma JM et al (2021) Cross-cohort generalizability of deep and conventional machine learning for MRI-based diagnosis and prediction of Alzheimer’s disease. NeuroImage: Clinical 31:1–9. https://doi.org/10.1016/j.nicl.2021.102712
Orouskhani M, Zhu C, Rostamian S, Zadeh FS, Shafiei M, Orouskhani Y (2022) Alzheimer’s disease detection from structural MRI using conditional deep triplet network. Neuroscience Informatics 2(4):1–7. https://doi.org/10.1016/j.neuri.2022.100066
Nguyen M, He T, An L, Alexander DC, Feng J, Yeo BTT (2020) Predicting Alzheimer’s disease progression using deep recurrent neural networks. Neuroimage 222:1–15. https://doi.org/10.1016/j.neuroimage.2020.117203
El-Geneedy M, Moustafa HED, Khalifa F, Khater H, AbdElhalim E (2023) An MRI-based deep learning approach for accurate detection of Alzheimer’s disease. Alexandria Eng J 63:211–221. https://doi.org/10.1016/j.aej.2022.07.062
Naz S, Ashraf A, Zaib A (2022) Transfer learning using freeze features for Alzheimer neurological disorder detection using ADNI dataset. Multimedia Syst 28(1):85–94. https://doi.org/10.1007/s00530-021-00797-3
Kumar LS, Hariharasitaraman S, Narayanasamy K, Thinakaran K, Mahalakshmi J, Pandimurugan V (2021) AlexNet approach for early stage Alzheimer’s disease detection from MRI brain images. Materials Today: Proceedings 51:1–8. https://doi.org/10.1016/j.matpr.2021.04.415
Gamal A, Elattar M, Selim S (2022) Automatic early diagnosis of alzheimer’s disease using 3D deep ensemble approach. IEEE Access 10:115974–115987. https://doi.org/10.1109/ACCESS.2022.3218621
Zhang J, Zheng B, Gao A, Feng X, Liang D, Long X (2021) A 3D densely connected convolution neural network with connection-wise attention mechanism for Alzheimer’s disease classification. Magn Reson Imaging 78:1–8. https://doi.org/10.1016/j.mri.2021.02.001
Shamrat FMJM, Akter S, Azam S, Karim A, Ghosh P, Tasnim Z, Hasib KM, De Boer F, Ahmed K (2023) AlzheimerNet: an effective deep learning based proposition for alzheimer’s disease stages classification from functional brain changes in magnetic resonance images. IEEE Access 11:16376–16395. https://doi.org/10.1109/ACCESS.2023.3244952
Fareed MMS, Zikria S, Ahmed G, Mui-Zzud-Din MS, Aslam M, Jillani SF, Moustafa A, Asad M (2022) ADD-Net: An effective deep learning model for early detection of alzheimer disease in MRI scans. IEEE Access 10:96930–96951. https://doi.org/10.1109/ACCESS.2022.3204395
Buvaneswari PR, Gayathri R (2021) Deep learning-based segmentation in classification of Alzheimer’s disease. Arab J Sci Eng 46(6):5373–5383. https://doi.org/10.1007/s13369-020-05193-z
Faisal FUR, Kwon GR (2022) Automated detection of alzheimer-s disease and mild cognitive impairment using whole brain MRI. IEEE Access 10:65055–65066. https://doi.org/10.1109/ACCESS.2022.3180073
Turkson RE, Qu H, Mawuli CB, Eghan MJ (2021) Classification of Alzheimer’s disease using deep convolutional spiking neural network. Neural Process Lett 53(4):2649–2663. https://doi.org/10.1007/s11063-021-10514-w
Bi X, Liu W, Liu H, Shang Q (2021) Artificial intelligence-based MRI images for brain in prediction of Alzheimer’s disease. J Healthcare Eng 2021:1–7. https://doi.org/10.1155/2021/8198552
Battineni G, Hossain MA, Chintalapudi N, Traini E, Dhulipalla VR, Ramasamy M, Amenta F (2021) Improved Alzheimer’s disease detection by MRI using multimodal machine learning algorithms. Diagnostics 11(11):1–15. https://doi.org/10.3390/diagnostics11112103
Sudharsan M, Thailambal G (2023) Alzheimer’s disease prediction using machine learning techniques and principal component analysis (PCA). Materials Today: Proceedings 81:182–190. https://doi.org/10.1016/j.matpr.2021.03.061
AlSaeed D, Omar SF (2022) Brain MRI analysis for Alzheimer’s disease diagnosis using CNN-based feature extraction and machine learning. Sensors 22(8):1–16. https://doi.org/10.3390/s22082911
Saratxaga CL, Moya I, Picon A, Acosta M, Moreno-Fernandez-de-Leceta A, Garrote E, Bereciartua-Perez A (2021) MRI deep learning-based solution for Alzheimer’s disease prediction. J Personalized Med 11(9):1–22. https://doi.org/10.3390/jpm11090902
Acknowledgements
We thank the anonymous referees for their useful suggestions.
Funding
This work has no funding resource.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by*1Haulath K, 2Mohamed Basheer K. P. The first draft of the manuscript was written by *1Haulath K and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Consent of publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haulath, K., Mohamed Basheer, K.P. An efficient GS-RBFN framework for early prediction and classification of ad. Multimed Tools Appl (2024). https://doi.org/10.1007/s11042-024-19168-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11042-024-19168-x