Abstract
Background
Escherichia coli is the most common etiological agent of urinary tract infections (UTIs). Meanwhile, plasmid-mediated quinolone resistance (PMQR) is reported in E. coli isolates producing extended-spectrum β-lactamases (ESBLs). Furthermore, the reservoirs and mechanisms of acquisition of uropathogenic Escherichia coli (UPEC) strains are poorly understood. On the other hand, UTIs are common in pregnant women and the treatment challenge is alarming.
Methods and results
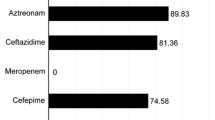
In the present study, 54 pregnant women with acute cystitis were included. A total of 108 E. coli isolates, 54 isolates from UTI and 54 isolates from faeces of pregnant women (same host) were collected. In the antimicrobial susceptibility test, the highest rate of antibiotic resistance was to nalidixic acid (77%, 83/108) and the lowest rate was to imipenem (9%, 10/108). Among the isolates, 44% (48/108) were ESBLs producers. A high frequency of PMQR genes was observed in the isolates. The frequency of PMQR genes qnrS, qnrB, aac(6′)-Ib-cr, and qnrA was 58% (63/108), 21% (23/108), 9% (10/108), and 4% (4/108), respectively. Meanwhile, PMQR genes were not detected in 24% (20/85) of isolates resistant to nalidixic acid and/or fluoroquinolone, indicating that other mechanisms, i.e. chromosomal mutations, are involved in resistance to quinolones, which were not detected in the present study. In ESBL-producing isolates, the frequency of PMQR genes was higher than that of non-ESBL-producing isolates (81% vs. 53%). Meanwhile, UTI and faeces isolates mainly belonged to phylogenetic group B2 (36/54, 67% and 25/54, 46%, respectively) compared to other phylogenetic groups. In addition, virulence factors and multidrug-resistant (MDR) were mainly associated with phylogenetic group B2. However, predominant clones in faeces were not found in UTIs. Rep-PCR revealed the presence of 85 clones in patients. Among the clones, 40 clones were detected only in faeces (faeces-only), 35 clones only in UTI (UTI-only) and 10 clones in both faeces and UTI (faeces-UTI). We found that out of 10 faeces-UTI clones, 5 clones were present in the host’s faeces flora.
Conclusion
This study revealed a high rate of resistance to the quinolone nalidixic acid and a widespread distribution of PMQR genes in MDR E. coli strains producing ESBLs. The strains represented virulence factors and phylogenetic group B2 are closely associated with abundance in UTI and faeces. However, the predominant clones in faeces were not found in UTIs and it is possible that rep-PCR is not sufficiently discriminating clones.

Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Klein RD, Hultgren SJ (2020) Urinary tract infections: microbial pathogenesis, host–pathogen interactions and new treatment strategies. Nat Rev Microbiol 18(4):211–226
Malekzadegan Y, Rastegar E, Moradi M, Heidari H, Sedigh Ebrahim-Saraie H (2019) Prevalence of quinolone-resistant uropathogenic Escherichia coli in a tertiary care hospital in south Iran [Response to letter]. Infect Drug Resist. :2175–2176
Sheerin NS (2011) Urinary tract infection. Medicine 39(7):384–389
Le J, Briggs GG, McKeown A, Bustillo G (2004) Urinary tract infections during pregnancy. Ann Pharmacother 38(10):1692–1701
Rahimi Z, Malekzadegan Y, Bahador A, Azimzadeh M, Haghighi MA (2022) Phylogenetic study, distribution of virulence genes and antibiotic resistance profiles of Escherichia coli isolated from Bushehr coastal water. Gene Rep 26:101473
Geerlings SE (2016) Clinical presentations and epidemiology of urinary tract infections. Microbiol Spectr 4(5):4
Vansofla AN, Nazarian S, Kordbache E, Fathi J (2021) An IgG/IgY sandwich-ELISA for the detection of heat-labile enterotoxin B subunit of enterotoxigenic Escherichia coli. Gene Rep 23:101099
Sarowska J, Futoma-Koloch B, Jama-Kmiecik A, Frej-Madrzak M, Ksiazczyk M, Bugla-Ploskonska G et al (2019) Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog 11:1–16
Terlizzi ME, Gribaudo G, Maffei ME (2017) UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol 8:1566
Yamamoto S, Tsukamoto T, Terai A, Kurazono H, Takeda Y, Yoshida O (1997) Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J Urol 157(3):1127–1129
Belete MA, Saravanan M (2020) A systematic review on drug resistant urinary tract infection among pregnant women in developing countries in Africa and Asia; 2005–2016. Infect Drug Resist. :1465–1477
Hadadi M, Malekzadegan Y, Heidari H, Sedigh Ebrahim-Saraie H, Motamedifar M (2016) Antimicrobial resistance pattern in Escherichia coli isolates obtained from a specialized women and children hospital in Shiraz, Iran: a prevalence study. J Health Sci Surveillance Syst 4(4):194–198
Rizvi M, Khan F, Shukla I, Malik A (2011) Rising prevalence of antimicrobial resistance in urinary tract infections during pregnancy: necessity for exploring newer treatment options. J Lab Physicians 3(02):098–103
Lee AC, Mullany LC, Koffi AK, Rafiqullah I, Khanam R, Folger LV et al (2020) Urinary tract infections in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth 20(1):1–11
Hassuna NA, Khairalla AS, Farahat EM, Hammad AM, Abdel-Fattah M (2020) Molecular characterization of extended-spectrum β lactamase-producing E. Coli recovered from community-acquired urinary tract infections in Upper Egypt. Sci Rep 10(1):2772
Pandit R, Awal B, Shrestha SS, Joshi G, Rijal BP, Parajuli NP (2020) Extended-spectrum β-lactamase (ESBL) genotypes among multidrug-resistant uropathogenic Escherichia coli clinical isolates from a teaching hospital of Nepal. Interdiscip Perspect Infect Dis. ;2020
Akgoz M, Akman I, Ates AB, Celik C, Keskin B, Ozmen Capin BB et al (2020) Plasmidic fluoroquinolone resistance genes in fluoroquinolone-resistant and/or extended spectrum beta-lactamase-producing Escherichia coli strains isolated from Pediatric and adult patients diagnosed with urinary tract infection. Microb Drug Resist 26(11):1334–1341
Pasom W, Chanawong A, Lulitanond A, Wilailuckana C, Kenprom S, Puang-Ngern P (2013) Plasmid-mediated quinolone resistance genes, aac (6′)-Ib-cr, qnrS, qnrB, and qnrA, in urinary isolates of Escherichia coli and Klebsiella pneumoniae at a teaching hospital, Thailand. Jpn J Infect Dis 66(5):428–432
Azargun R, Gholizadeh P, Sadeghi V, Hosainzadegan H, Tarhriz V, Memar MY et al (2020) Molecular mechanisms associated with quinolone resistance in Enterobacteriaceae: review and update. Trans R Soc Trop Med Hyg 114(10):770–781
Nordmann P, Poirel L (2005) Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother 56(3):463–469
Jacoby GA, Strahilevitz J, Hooper DC (2015) Plasmid-mediated quinolone resistance. Plasmids: Biology and Impact in Biotechnology and Discovery. :475–503
Mahon CR, Lehman DC (2022) Textbook of diagnostic microbiology-e-book. Elsevier Health Sciences
Weinstein M, Patel J, Bobenchik A, Campeau S, Cullen S, Galas M et al (2020) M100 Performance standards for Antimicrobial susceptibility testing a CLSI supplement for global application. Performance standards for antimicrobial susceptibility testing performance standards for antimicrobial susceptibility testing
Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas M, Giske C et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281
Harwalkar A, Sataraddi J, Gupta S, Yoganand R, Rao A, Srinivasa H (2013) The detection of ESBL-producing Escherichia coli in patients with symptomatic urinary tract infections using different diffusion methods in a rural setting. J Infect Public Health 6(2):108–114
Clermont O, Bonacorsi S, Bingen E (2000) Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66(10):4555–4558
Snelling AM, Gerner-Smidt P, Hawkey PM, Heritage J, Parnell P, Porter C et al (1996) Validation of use of whole-cell repetitive extragenic palindromic sequence-based PCR (REP-PCR) for typing strains belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii complex and application of the method to the investigation of a hospital outbreak. J Clin Microbiol 34(5):1193–1202
Sato T, Yokota S-i, Uchida I, Okubo T, Usui M, Kusumoto M et al (2013) Fluoroquinolone resistance mechanisms in an Escherichia coli isolate, HUE1, without quinolone resistance-determining region mutations. Front Microbiol 4:125
Fallah F, Parhiz S, Azimi L (2019) Distribution and antibiotic resistance pattern of bacteria isolated from patients with community-acquired urinary tract infections in Iran: a cross-sectional study. Int J Health Sci. ;4(2)
Sultana KF, Akter A, Saha SR, Ahmed F, Alam S, Jafar T et al (2023) Bacterial profile, antimicrobial resistance, and molecular detection of ESBL and quinolone resistance gene of uropathogens causing urinary tract infection in the southeastern part of Bangladesh. Braz J Microbiol. :1–13
Pitout JD (2012) Extraintestinal pathogenic Escherichia coli: a combination of virulence with antibiotic resistance. Front Microbiol 3:9
Mohajeri P, Darfarin G, Farahani A (2014) Genotyping of ESBL producing Uropathogenic Escherichia coli in west of Iran. Int J Microbiol. ;2014
Kakian F, Fathi J, Alvandi F, Moumivand M, Gholami AR, Gholipour A et al (2020) The occurrence of antibiotic resistance, ESBLs, MBL and NDM-1 in Uropathogenic Escherichia coli in Central part of Iran. J Curr Biomed Rep 1(2):73–76
Baziboroun M, Bayani M, Poormontaseri Z, Shokri M, Biazar T (2018) Prevalence and antibiotic susceptibility pattern of extended spectrum beta lactamases producing Escherichia coli isolated from outpatients with urinary tract infections in Babol, Northern of Iran. Curr Issues Pharm Med Sci 31(2):61–64
Farzi S, Ranjbar R, Niakan M, Ahmadi MH (2021) Molecular characterization of Antibiotic Resistance Associated with TEM and CTX-M ESBL in Uropathogenic E. coli strains isolated from outpatients. Iran J Pathol 16(4):386
Jena J, Sahoo RK, Debata NK, Subudhi E (2017) Prevalence of TEM, SHV, and CTX-M genes of extended-spectrum β-lactamase-producing Escherichia coli strains isolated from urinary tract infections in adults. 3 Biotech 7:1–7
Kim HB, Park CH, Kim CJ, Kim E-C, Jacoby GA, Hooper DC (2009) Prevalence of plasmid-mediated quinolone resistance determinants over a 9-year period. Antimicrob Agents Chemother 53(2):639–645
FarajzadehSheikh A, Veisi H, Shahin M, Getso M, Farahani A (2019) Frequency of quinolone resistance genes among extended-spectrum β-lactamase (ESBL)-producing Escherichia coli strains isolated from urinary tract infections. Trop Med Health 47:1–7
Hassan W, Hashim A, Domany R (2012) Plasmid mediated quinolone resistance determinants qnr, aac (6′)-Ib-cr, and qep in ESBL-producing Escherichia coli clinical isolates from Egypt. Indian J Med Microbiol. ;30(4):442-7
Ramakrishnan V, Marialouis XA, Al-Ansari MM, Al-Humaid L, Santhanam A, Obulisamy PK (2022) Multilocus sequence typing and ERIC-PCR fingerprinting of virulent clinical isolates of uropathogenic multidrug resistant Escherichia coli. J King Saud Univ Sci 34(3):101874
Nielsen KL, Dynesen P, Larsen P, Frimodt-Møller N (2014) Faecal Escherichia coli from patients with E. coli urinary tract infection and healthy controls who have never had a urinary tract infection. J Med Microbiol. ;63(4):582-9
Rezatofighi SE, Mirzarazi M, Salehi M (2021) Virulence genes and phylogenetic groups of uropathogenic Escherichia coli isolates from patients with urinary tract infection and uninfected control subjects: a case-control study. BMC Infect Dis 21:1–11
Lee J, Subhadra B, Son YJ, Kim D, Park H, Kim J et al (2016) Phylogenetic group distributions, virulence factors and antimicrobial resistance properties of uropathogenic Escherichia coli strains isolated from patients with urinary tract infections in South Korea. Lett Appl Microbiol 62(1):84–90
Molina-López J, Aparicio-Ozores G, Ribas-Aparicio RM, Gavilanes-Parra S, Chávez-Berrocal ME, Hernández-Castro R et al (2011) Drug resistance, serotypes, and phylogenetic groups among uropathogenic Escherichia coli including O25-ST131 in Mexico City. J Infect Dev Ctries 5(12):840–849
Johnson JR, Brown JJ, Carlino UB, Russo TA (1998) Colonization with and acquisition of uropathogenic Escherichia coli as revealed by polymerase chain reaction-based detection. J Infect Dis 177(4):1120–1124
Foxman B, Zhang L, Tallman P, Andree BC, Geiger AM, Koopman JS et al (1997) Transmission of uropathogens between sex partners. J Infect Dis 175(4):989–992
Martinson JN, Walk ST (2020) Escherichia coli residency in the gut of healthy human adults. EcoSal Plus 9(1). https://doi.org/10.1128/ecosalplusESP-0003-2020
Moreno E, Andreu A, Pigrau C, Kuskowski MA, Johnson JR, Prats G (2008) Relationship between Escherichia coli strains causing acute cystitis in women and the fecal E. Coli population of the host. J Clin Microbiol 46(8):2529–2534
Hashemizadeh Z, Kalantar-Neyestanaki D, Mansouri S (2017) Association between virulence profile, biofilm formation and phylogenetic groups of Escherichia coli causing urinary tract infection and the commensal gut microbiota: a comparative analysis. Microb Pathog 110:540–545
Hashemizadeh Z, Kalantar-Neyestanaki D, Mansouri S (2018) Clonal relationships, antimicrobial susceptibilities, and molecular characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates from urinary tract infections and fecal samples in Southeast Iran. Rev Soc Bras Med Trop 51:44–51
Chen X, Zhang W, Pan W, Yin J, Pan Z, Gao S et al (2012) Prevalence of qnr, aac (6′)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob Agents Chemother. ;56(6):3423-7
Acknowledgements
The authors would like to thank the Research and Technology Vice-Chancellor of Shiraz University of Medical Sciences (No. IR.SUMS.REC. 1398.959) for financial support.
Funding
This research was supported by the Research and Technology Vice-Chancellor of Shiraz University of Medical Sciences (No. IR.SUMS.REC. 1398.959).
Author information
Authors and Affiliations
Contributions
MS and Jf wrote the manuscript. SM, Jf and ZH designed the study. SKH and MH collected the samples. ZH performed the phenotypic and genotypic tests. MS, ZH, SM and ZD analyzed the data. ZH supervised the project and wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (No. IR.SUMS.REC. 1398.959). Written informed consent was obtained from each participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sohrabi, M., Fathi, J., Mohebi, S. et al. High prevalence of plasmid-mediated quinolone resistance in escherichia coli strains producing extended-spectrum beta-lactamases isolated from faeces and urine of pregnant women with acute cystitis. Mol Biol Rep 51, 566 (2024). https://doi.org/10.1007/s11033-024-09491-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09491-9