Abstract
Purpose
Crohn’s disease is a chronic gastrointestinal inflammatory disease with possible extraintestinal symptoms. There are predisposing genetic factors and even monogenic variants of the disorder. One of the possible genetic factors are variants of the DUOX2 gene. The protein product of the DUOX2 gene is a dual oxidase enzyme producing H2O2 in the bowel. Reduced H2O2 levels impact mucosal homeostasis and contribute to the development of inflammatory bowel disease. Thus far, only 19 patients with IBD with the DUOX2 variants have been described.
Methods
Here we present a case report of an adolescent female diagnosed at eleven years of age with IBD that was subsequently reclassified as Crohn’s disease. She was treated with immunosuppressants and biological therapy but experienced additional complications. Her peripheral blood lymphocyte DNA was studied using massive parallel sequencing. Detected variants were functionally studied.
Results
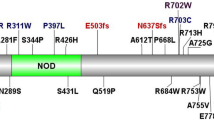
Whole exome sequencing found two novel DUOX2 gene variants: a de novo variant c.3646C>T; p.R1216W and a maternally inherited variant c.3391G>A; p.A1131T which were initially classified as variants of unknown significance. However, follow-up functional studies demonstrated that both DUOX2 variants led to impaired H2O2 generation, which led to their reclassification to the likely pathogenic class according to the ACMG.net. Therefore, we conclude that these variants are causative for the disease.
Conclusions
Identifying novel variants in patients with Crohn’s disease and their families is important for precision medicine approaches and understanding of the pathogenesis of likely “monogenic” rare forms of inflammatory bowel disease.

Similar content being viewed by others
Data availability
The datasets used and/or analyzed during this study are available from the corresponding author upon request.
References
Ashton JJ, Ennis S, Beattie RM (2017) Early-onset paediatric inflammatory bowel disease. Lancet Child Adolesc Health 1:147–158. https://doi.org/10.1016/S2352-4642(17)30017-2
Uhlig HH, Charbit-Henrion F, Kotlarz D et al (2021) Clinical Genomics for the diagnosis of monogenic forms of inflammatory bowel disease: a position paper from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 72:456–473. https://doi.org/10.1097/MPG.0000000000003017
Levine AP, Pontikos N, Schiff ER et al (2016) Genetic complexity of Crohn’s Disease in two large Ashkenazi jewish families. Gastroenterology 151:698–709. https://doi.org/10.1053/j.gastro.2016.06.040
Hayes P, Dhillon S, O’Neill K et al (2015) Defects in nicotinamide-adenine dinucleotide phosphate oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol 1:489–502. https://doi.org/10.1016/j.jcmgh.2015.06.005
Parlato M, Charbit-Henrion F, Hayes P et al (2017) First Identification of Biallelic inherited DUOX2 inactivating mutations as a cause of very early onset inflammatory bowel disease. Gastroenterology 153:609–611e3. https://doi.org/10.1053/j.gastro.2016.12.053
Kyodo R, Takeuchi I, Narumi S et al (2022) Novel biallelic mutations in the DUOX2 gene underlying very early-onset inflammatory bowel disease: a case report. Clin Immunol 238:109015. https://doi.org/10.1016/j.clim.2022.109015
Grasberger H, De Deken X, Miot F et al (2007) Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol 21:1408–1421. https://doi.org/10.1210/me.2007-0018
El Hassani RA, Benfares N, Caillou B et al (2005) Dual oxidase2 is expressed all along the digestive tract. Am J Physiology-Gastrointestinal Liver Physiol 288:G933–G942. https://doi.org/10.1152/ajpgi.00198.2004
Deken XD, Wang D, Many M-C et al (2000) Cloning of two human thyroid cDNAs encoding New members of the NADPH oxidase family *. J Biol Chem 275:23227–23233. https://doi.org/10.1074/jbc.M000916200
Stenke E, Bourke B, Knaus UG (2019) NADPH oxidases in inflammatory bowel disease. In: Knaus UG, Leto TL (eds) NADPH oxidases: methods and protocols. Springer, New York, NY, pp 695–713
Grasberger H, Refetoff S (2006) Identification of the maturation factor for dual oxidase: evolution of an eukaryotic operon equivalent. J Biol Chem 281:18269–18272. https://doi.org/10.1074/jbc.C600095200
Tangye SG, Al-Herz W, Bousfiha A et al (2020) Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 40:24–64. https://doi.org/10.1007/s10875-019-00737-x
Liu H, Naismith JH (2008) An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol 8:91. https://doi.org/10.1186/1472-6750-8-91
Rigutto S, Hoste C, Grasberger H et al (2009) Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem 284:6725–6734. https://doi.org/10.1074/jbc.M806893200
Morand S, Ueyama T, Tsujibe S et al (2009) Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J 23:1205–1218. https://doi.org/10.1096/fj.08-120006
Zamproni I, Grasberger H, Cortinovis F et al (2008) Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a Novel cause of congenital hypothyroidism. J Clin Endocrinol Metab 93:605–610. https://doi.org/10.1210/jc.2007-2020
Richards S, Aziz N, Bale S et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Sci 17(5):405–423. https://doi.org/10.1038/gim.2015.30
Grasberger H, Magis AT, Sheng E et al (2021) DUOX2 variants associate with preclinical disturbances in microbiota-immune homeostasis and increased inflammatory bowel disease risk. J Clin Invest 131:e141676. https://doi.org/10.1172/JCI141676
Lipinski S, Till A, Sina C et al (2009) DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 122:3522–3530. https://doi.org/10.1242/jcs.050690
Burgueño JF, Fritsch J, González EE et al (2021) Epithelial TLR4 signaling activates DUOX2 to Induce Microbiota-Driven Tumorigenesis. Gastroenterology 160:797–808e6. https://doi.org/10.1053/j.gastro.2020.10.031
Acknowledgements
We thank Doctor Ulla G. Knaus and Professor Helmut Grasberger for providing plasmids used in the study. We want to thank Tom Secrest for English proofreading. We would also like to extend our gratitude to the patient’s family for their cooperation. This work was done under the auspices of ERN ITHACA.
Funding
Ministry of Health, Czech Republic [grant number 00064203]; Ministry of Youth Education and Sports, Czech Republic [grant number LM2018132] and the Charles University, Czech Republic [grant number GAUK 134121].
Author information
Authors and Affiliations
Contributions
Author Martin Schwarz and Matej Gazdarica contributed equally and share co-first authorship. Matej Gazdarica conceived, performed and interpreted functional studies and drafted their respective parts in the manuscript. Martin Schwarz, conceived, designed and drafted the rest of the manuscript, led genetic consultations with the patients and interpreted laboratory genetic testing results. Eva Froňková, Michael Svatoň and Anna Křepelová performed and interpreted laboratory genetic testing results. Jiří Bronský, Markéta Havlovicová, Milan Macek jr. substantively revised the manuscript. Jiří Bronský followed the patient in the pediatric gastroenterology clinic. All authors have agreed to be personally accountable for their contributions. All authors read and approved the final manuscript. The manuscript, including related data, figures and tables has not been previously published and that the manuscript is not under consideration elsewhere.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflict of interest which could affect the content or outcomes reported in this manuscript.
Informed consent
The study was approved by the Internal Ethics Board of the Motol University Hospital and was carried out in line with the Declaration of Helsinki principles. Parents of the patient underwent genetic counseling and signed informed consent with research and publication of its results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schwarz, M., Gazdarica, M., Froňková, E. et al. Functional studies associate novel DUOX2 gene variants detected in heterozygosity to Crohn’s disease. Mol Biol Rep 51, 399 (2024). https://doi.org/10.1007/s11033-024-09317-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09317-8