Abstract
Background
Subarachnoid hemorrhage (SAH) is one of the most prevalent brain injuries in humans which has poor prognosis and high mortality rates. Due to several medical or surgical treatment methods, a gold standard method doesn’t exist for SAH treatment. Piceatannol (PCN), a natural analog of resveratrol, was reported to reduce inflammation and apoptosis promising a wide range of therapeutic alternatives. In this study, we aimed to investigate the effects of PCN in an experimental SAH model. The alleviating effects of PCN in the hippocampus in an experimental SAH model were investigated for the first time.
Methods and results
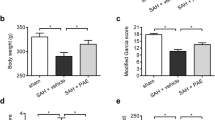
In this study, 27 Wistar Albino male rats (200–300 g; 7–8 week) were used. Animals were divided into three groups; SHAM, SAH, and SAH + PCN. SAH model was created with 120 µl of autologous arterial tail blood to prechiasmatic cisterna. 30 mg/kg PCN was administered intraperitoneally at 1st h after SAH. Neurological evaluation was performed with Garcia's score. RT-PCR was performed for gene expression levels in the hippocampus. Pyknosis, edema, and apoptosis were evaluated by H&E and TUNEL staining. Our results indicated that PCN administration reduced apoptosis (P < 0.01), cellular edema, and pyknosis (P < 0.05) in the hippocampus after SAH. Moreover, PCN treatment significantly decreased the expression levels of TNF-α (P < 0.01), IL-6 (P < 0.05), NF-κB (P < 0.05), and Bax (P < 0.05) in the hippocampus.
Conclusions
Our results demonstrated that PCN might be a potential therapeutic adjuvant agent for the treatment of early brain injury (EBI) following SAH. Further studies are required to clarify the underlying mechanisms and treatment options of SAH.





Similar content being viewed by others
Data availability
The datasets are available from the corresponding author upon reasonable request.
References
Long B, Koyfman A, Runyon MS (2017) Subarachnoid hemorrhage: updates in diagnosis and management. Emerg Med Clin North Am 35:803–824. https://doi.org/10.1016/J.EMC.2017.07.001
Simon M, Grote A (2021) Interleukin 6 and aneurysmal subarachnoid haemorrhage. A narrative review. Int J Mol Sci 22:4133
Sen SS, Rai SN, Birla H et al (2020) NF-κB-mediated neuroinflammation in parkinson’s disease and potential therapeutic effect of polyphenols. Neurotox Res 37:491–507. https://doi.org/10.1007/S12640-019-00147-2
Wang YC, Wang PF, Fang H et al (2013) Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44:2545–2552. https://doi.org/10.1161/STROKEAHA.113.001038/-/DC1
Edebali N, Tekin IÖ, Açıkgöz B, Açıkgöz S, Barut F, Sevinç N, Sümbüloğlu V et al (2014) Apoptosis and necrosis in the circumventricular organs after experimental subarachnoid hemorrhage as detected with annexin V and caspase 3 immunostaining. Neurol Res 36:1114–1120. https://doi.org/10.1179/1743132814Y.0000000437
He X, Sun J, Huang X (2018) Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp Ther Med 15:873–877. https://doi.org/10.3892/ETM.2017.5438
Nijhawan D, Honarpour N, Wang X (2000) Apoptosis in neural development and disease. Annu Rev Neurosci 23:73–87. https://doi.org/10.1146/ANNUREV.NEURO.23.1.73
Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81:471–483
Zhou Y, Khan H, Hoi MPM, Cheang WS (2022) Piceatannol protects brain endothelial cell line (bEnd.3) against lipopolysaccharide-induced inflammation and oxidative stress. Molecules. https://doi.org/10.3390/molecules27041206
Khan I, Preeti K, Kumar R et al (2023) Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int Immunopharmacol 116:109793. https://doi.org/10.1016/J.INTIMP.2023.109793
Yuan B, Zhao X-D, Shen J-D et al (2022) Activation of SIRT1 alleviates ferroptosis in the early brain injury after subarachnoid hemorrhage. Oxid Med Cell Longev. https://doi.org/10.1155/2022/9069825
Akar A, Öztopuz RÖ, Büyük B et al (2023) Neuroprotective effects of piceatannol on olfactory bulb injury after subarachnoid hemorrhage. Mol Neurobiol 60:3695–3706. https://doi.org/10.1007/S12035-023-03306-X
Lynch Donald DG, Zucker B, Shah KA et al (2023) Neurobehavioral impairments predict specic cerebral damage in rat model of subarachnoid hemorrhage. Res Sq. https://doi.org/10.21203/rs.3.rs-2943917/v1
Shi X, Fu L (2019) Piceatannol inhibits oxidative stress through modification of Nrf2-signaling pathway in testes and attenuates spermatogenesis and steroidogenesis in rats exposed to cadmium during adulthood. Drug Des Dev Ther 13:2811–2824. https://doi.org/10.2147/DDDT.S198444
Hao H, Bai Y, Liu Y et al (2021) Protective mechanism of FoxO1 against early brain injury after subarachnoid hemorrhage by regulating autophagy. Brain Behav. https://doi.org/10.1002/brb3.2376
Malçok ÜA, Büyük B (2021) Investigation of the neuroprotective effect of melatonin on hippocampal neuron injury developing due to the neurotoxic effect of cisplatin. Izmir Democr Univ Health Sci J 4:87–93. https://doi.org/10.52538/IDUHES.926453
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/METH.2001.1262
Setoguchi Y, Oritani Y, Ito R et al (2014) Absorption and metabolism of piceatannol in rats. J Agric Food Chem 62:2541–2548. https://doi.org/10.1021/JF404694Y
Ieong C, Sun H, Wang Q, Ma J (2018) Glycyrrhizin suppresses the expressions of HMGB1 and ameliorates inflammative effect after acute subarachnoid hemorrhage in rat model. J Clin Neurosci 47:278–284. https://doi.org/10.1016/J.JOCN.2017.10.034
Wang L, Guo Y, Ye J et al (2021) Protective effect of piceatannol against cerebral ischaemia-reperfusion injury via regulating Nrf2/HO-1 pathway in vivo and vitro. Neurochem Res 46:1869–1880. https://doi.org/10.1007/S11064-021-03328-8
He Y, Xu L, Li B et al (2015) Macrophage-inducible C-type lectin/spleen tyrosine kinase signaling pathway contributes to neuroinflammation after subarachnoid hemorrhage in rats. Stroke 46:2277–2286. https://doi.org/10.1161/STROKEAHA.115.010088
Maddahi A, Povlsen G, Edvinsson L (2012) Regulation of enhanced cerebrovascular expression of proinflammatory mediators in experimental subarachnoid hemorrhage via the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. J Neuroinflamm. https://doi.org/10.1186/1742-2094-9-274
Zaremba J, Losy J (2004) Cytokines in clinical and experimental ischemic stroke. Neurol Neurochir Pol 38:57–62
Ye SM, Johnson RW (2001) Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor κB. J Neuroimmunol 117:87–96. https://doi.org/10.1016/S0165-5728(01)00316-2
Bulters D, Gaastra B, Zolnourian A et al (2018) Haemoglobin scavenging in intracranial bleeding: biology and clinical implications. Nat Rev Neurol 14:416–432. https://doi.org/10.1038/S41582-018-0020-0
Hyzak L, Moos R, Von Rath F et al (2011) Quantitative matrix-assisted laser desorption ionization-time-of-flight mass spectrometry analysis of synthetic polymers and peptides. Anal Chem 83:9467–9471. https://doi.org/10.1021/AC2021739
Peng LY, Yuan M, Shi HT et al (2020) Protective effect of piceatannol against acute lung injury through protecting the integrity of air-blood barrier and modulating the TLR4/NF-κB signaling pathway activation. Front Pharmacol. https://doi.org/10.3389/FPHAR.2019.01613
Du SH, Yang MY, Gan HL et al (2023) Piceatannol-3’-O-β-d-glucopyranoside alleviates nephropathy via regulation of High mobility group B-1 (HMGB1)/Toll-like receptor 4 (TLR4)/Nuclear factor kappa B (NF-κB) signalling pathway. J Pharm Pharmacol 75:693–702. https://doi.org/10.1093/JPP/RGAD021
Zhang Y, Yang X, Ge X, Zhang F (2019) Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed Pharmacother 109:726–733. https://doi.org/10.1016/J.BIOPHA.2018.10.161
Zhou XY, Sun JY, Wang WQ et al (2022) TAT-HSP27 peptide improves neurologic deficits via reducing apoptosis after experimental subarachnoid hemorrhage. Front Cell Neurosci. https://doi.org/10.3389/FNCEL.2022.878673
Guo D, Xie J, Zhao J et al (2018) Resveratrol protects early brain injury after subarachnoid hemorrhage by activating autophagy and inhibiting apoptosis mediated by the Akt/mTOR pathway. NeuroReport 29:368–379. https://doi.org/10.1097/WNR.0000000000000975
Cahill J, Calvert JW, Marcantonio S, Zhang JH (2007) p53 may play an orchestrating role in apoptotic cell death after experimental subarachnoid hemorrhage. Neurosurgery 60:531–545. https://doi.org/10.1227/01.NEU.0000249287.99878.9B
Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22:9030–9040. https://doi.org/10.1038/SJ.ONC.1207116
Chen X, Chen C, Fan S et al (2018) Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflamm. https://doi.org/10.1186/S12974-018-1151-3
Vellimana AK, Aum DJ, Diwan D et al (2020) SIRT1 mediates hypoxic preconditioning induced attenuation of neurovascular dysfunction following subarachnoid hemorrhage. Exp Neurol. https://doi.org/10.1016/J.EXPNEUROL.2020.113484
Acknowledgements
This article was partly produced from the master of science thesis of the first author.
Funding
This work was supported by the Scientific Research Coordination Unit [BAPD] of Çanakkale Onsekiz Mart University, Çanakkale, Turkey (Project Number: TYL-2021-3406).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this study.
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare that there is no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Çanakkale Onsekiz Mart University, Turkey. (Decision number protocol number: 2020-E.2000168654).
Consent to participate
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Erbil, G., Uzun, M. Investigation of the protective effects of piceatannol on experimental subarachnoid hemorrhage in rats. Mol Biol Rep 51, 366 (2024). https://doi.org/10.1007/s11033-024-09275-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09275-1