Abstract
Background
Spondyloepimetaphyseal dysplasia with joint laxity type 3 (SEMDJL3) is a rare skeletal dysplasia associated with EXOC6B, a component of the exocyst complex, involved in vesicle tethering and exocytosis at the plasma membrane. So far, EXOC6B and the pathomechanisms underlying SEMDJL3 remain obscure.
Methods and results
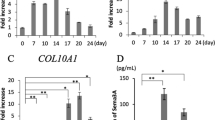
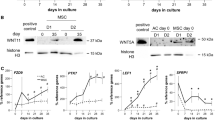
Exoc6b was detected largely at the perinuclear regions and the primary cilia base in ATDC5 prechondrocytes. Its shRNA lentiviral knockdown impeded primary ciliogenesis. In Exoc6b silenced prechondrocytes, Hedgehog signaling was attenuated, including when stimulated with Smoothened agonist. Exoc6b knockdown deregulated the mRNA and protein levels of Col2a1, a marker of chondrocyte proliferation at 7- and 14-days following differentiation. It led to the upregulation of Ihh another marker of proliferative chondrocytes. The levels of Col10a1, a marker of chondrocyte hypertrophy was enhanced at 14 days of differentiation. Congruently, Axin2, a canonical Wnt pathway modulator that inhibits chondrocyte hypertrophy was repressed. The expression of Mmp13 and Adamts4 that are terminal chondrocyte hypertrophy markers involved in extracellular matrix (ECM) remodelling were downregulated at 7 and 14 days of chondrogenesis. Bglap that encodes for the most abundant non-collagenous bone matrix constituent and promotes ECM calcification was suppressed at 14 days of chondrocyte differentiation. ECM mineralization was assessed by Alizarin Red staining. Gene expression and ciliogenesis were investigated by reverse transcription quantitative real-time PCR, immunoblotting, and immunocytochemistry.
Conclusions
These findings provide initial insights into the potential role of Exoc6b in primary ciliogenesis and chondrogenic differentiation, contributing towards a preliminary understanding of the molecular pathomechanisms underlying SEMDJL3.






Similar content being viewed by others
Data availability
The data presented in this study are available upon request from the corresponding author.
Abbreviations
- SEMDJL3:
-
Spondyloepimetaphyseal dysplasia with joint laxity, type 3
- MTC:
-
Multisubunit tethering complexes
- SNARE:
-
N-Ethylmaleimide-sensitive factor attachment protein receptors
- GTPases:
-
Guanosine triphosphatases
- CATCHR:
-
Complexes associated with tethering containing helical rods
- ERK1/2:
-
Extracellular signal-regulated kinase 1 and 2
- PI(4,5)P2 :
-
Phosphatidylinositol 4,5 biphosphate
- IFT:
-
Intraflagellar transport
- KD:
-
Knockdown
- SAG:
-
Smoothened agonist
- Col2a1:
-
Type II collagen alpha 1
- Hh:
-
Hedgehog
- Ihh:
-
Indian hedgehog
- Col10a1:
-
Type X collagen alpha 1
- Mmp13:
-
Matrix metallopeptidase 13
- Adamts4:
-
ADAM metallopeptidase with thrombospondin type 1 Motif 4
- Bglap:
-
Bone-gamma carboxyglutamate protein
- DMEM:
-
Dulbecco’s modified eagle medium
- FBS:
-
Fetal bovine serum
- PFA:
-
Paraformaldehyde
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- PBS:
-
Phosphate-buffered saline
- shRNA:
-
Short-hairpin RNA
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription PCR
- RT-qPCR:
-
Reverse transcription quantitative real time PCR
- SDS-PAGE:
-
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- RT:
-
Room temperature
- SEM:
-
Standard error of the mean
- ANOVA:
-
Analysis of variance
- ns:
-
Non-significant
References
Lurick A, Kummel D, Ungermann C (2018) Multisubunit tethers in membrane fusion. Curr Biol 28(8):R417–R420
Wu B, Guo W (2015) The exocyst at a glance. J Cell Sci 128(16):2957–2964
Grindstaff KK et al (1998) Section 6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93(5):731–740
Prigent M et al (2003) ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J Cell Biol 163(5):1111–1121
He B et al (2007) Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 26(18):4053–4065
Zhang X et al (2008) Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol 180(1):145–158
Guo W et al (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18(4):1071–1080
Ahmed SM et al (2018) Exocyst dynamics during vesicle tethering and fusion. Nat Commun 9(1):5140
Gromley A et al (2005) Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell 123(1):75–87
Hehnly H et al (2012) The centrosome regulates the Rab11- dependent recycling endosome pathway at appendages of the mother centriole. Curr Biol 22(20):1944–1950
Schmidt KN et al (2012) Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 199(7):1083–1101
Knodler A et al (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A 107(14):6346–6351
Park TJ et al (2008) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40(7):871–879
Girisha KM et al (2016) A novel multiple joint dislocation syndrome associated with a homozygous nonsense variant in the EXOC6B gene. Eur J Hum Genet 24(8):1206–1210
Campos-Xavier B et al (2018) Confirmation of spondylo-epi-metaphyseal dysplasia with joint laxity, EXOC6B type. Am J Med Genet A 176(12):2934–2935
Simsek-Kiper PO et al (2022) Biallelic loss-of-function variants in EXOC6B are associated with impaired primary ciliogenesis and cause spondylo-epi-metaphyseal dysplasia with joint laxity type 3. Hum Mutat 43:2116
Zhang XM et al (2004) Section 15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 279(41):43027–43034
Wu S et al (2005) Section 15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12(10):879–885
Jin Y et al (2011) Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex. Dev Cell 21(6):1156–1170
Feng S et al (2012) A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem 287(19):15602–15609
Upadhyai P, Guleria VS, Udupa P (2020) Characterization of primary cilia features reveal cell-type specific variability in in vitro models of osteogenic and chondrogenic differentiation. PeerJ 8:e9799
Quadri N, Upadhyai P (2023) Primary cilia in skeletal development and disease. Exp Cell Res 431(1):113751
Zhao Q et al (1997) Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn 209(4):377–386
St-Jacques B, Hammerschmidt M, McMahon AP (1999) Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13(16):2072–2086
Lu Y et al (2014) Col10a1 gene expression and chondrocyte hypertrophy during skeletal development and disease. Front Biol 9(3):195–204
Dao DY et al (2010) Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res 28(1):89–95
Inada M et al (2004) Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A 101(49):17192–17197
Djouad F et al (2007) Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther 9(2):R33
Han Y et al (2016) Leptin induces osteocalcin expression in ATDC5 cells through activation of the MAPK-ERK1/2 signaling pathway. Oncotarget 7(39):64021–64029
Wehrle A et al (2018) A common pathomechanism in GMAP-210- and LBR-related diseases. JCI Insight 3(23):e121150
Wehrle A et al (2019) Hypomorphic mutations of TRIP11 cause odontochondrodysplasia. JCI Insight 4(3):e124701
Yeaman C et al (2001) Sec6/8 complexes on trans-golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol 155(4):593–604
Shin DM et al (2000) The mammalian Sect6/8 complex interacts with ca(2+) signaling complexes and regulates their activity. J Cell Biol 150(5):1101–1112
Rogers KK et al (2004) The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun 319(1):138–143
Seixas C et al (2016) Arl13b and the exocyst interact synergistically in ciliogenesis. Mol Biol Cell 27(2):308–320
Zuo X et al (2019) The exocyst acting through the primary cilium is necessary for renal ciliogenesis, cystogenesis, and tubulogenesis. J Biol Chem 294(17):6710–6718
Zuo X, Guo W, Lipschutz JH (2009) The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20(10):2522–2529
Fogelgren B et al (2011) The exocyst protein Sec10 interacts with polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7(4):e1001361
Wang C, Yuan X, Yang S (2013) IFT80 is essential for chondrocyte differentiation by regulating hedgehog and wnt signaling pathways. Exp Cell Res 319(5):623–632
Baek J-I et al (2016) Dynamin binding protein (Tuba) deficiency inhibits ciliogenesis and nephrogenesis in vitro and in vivo*. J Biol Chem 291(16):8632–8643
Zuo X, Fogelgren B, Lipschutz JH (2011) The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells*. J Biol Chem 286(25):22469–22477
Collins I, Wann AKT (2020) Regulation of the extracellular matrix by ciliary machinery. Cells 9(2):1
Pereira C et al (2023) The exocyst complex is an essential component of the mammalian constitutive secretory pathway. J Cell Biol 222(5):e202205137
Yamaguchi H et al (2022) Temporospatial regulation of intraflagellar transport is required for the endochondral ossification in mice. Dev Biol 482:91–100
Haycraft CJ et al (2007) Intraflagellar transport is essential for endochondral bone formation. Development 134(2):307–316
McGlashan SR et al (2007) Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol 26(4):234–246
Song B et al (2007) Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol 305(1):202–216
Tummala P, Arnsdorf EJ, Jacobs CR (2010) The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng 3(3):207–212
Lian C et al (2019) Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin beta1-SMAD1 interaction. Bone Res 7:8
Edfors F et al (2016) Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol Syst Biol 12(10):883
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165(3):535–550
Dong YF et al (2006) Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol 208(1):77–86
He X (2008) Cilia put a brake on wnt signalling. Nat Cell Biol 10(1):11–13
Martin-Urdiroz M et al (2016) The exocyst complex in health and disease. Front Cell Dev Biol 4:24
Lim JE et al (2005) A mutation in Sec15l1 causes anemia in hemoglobin deficit (hbd) mice. Nat Genet 37(11):1270–1273
Acknowledgements
We are grateful to Dr. Uwe Kornak, Charité—Universitätsmedizin Berlin for ATDC5 cells.
Funding
This work was supported by an Early Career Research award (ECR/2016/001475) for the project entitled ‘Investigating the role of Ift52 and Exoc6B in human biology and disease using cell culture and Drosophila systems’ from the Science and Engineering Research Board, Department of Science and Technology, Government of India and the project entitled ‘Investigating the crosstalk between primary cilia and autophagy in chondrogenesis and its modulation by Fibroblast growth factor (FGF) signaling in FGFR3 related skeletal dysplasias in vitro’ (2020 -107/CMB/ADHOC-BMS) funded by the Indian Council for Medical Research, Government of India to Priyanka Upadhyai.
Author information
Authors and Affiliations
Contributions
PU: Conceptulization, Methodology, Resources, Validation, Formal Analysis, Visualization, Writing-original draft, Writing-review and editing, Supervision, Funding acquisition, Project administration. VSG: Methodology, Investigation, Validation, Visualization, Data curation, Formal Analysis, Writing-original draft. NQ: Methodology, Investigation, Validation, Visualization, Data curation, Formal Analysis, Writing-review and editing. KP: Investigation, Validation, Visualization. RD: Formal Analysis, Validation, Visualization, Writing-review and editing. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Research involving human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guleria, V.S., Quadri, N., Prasad, K. et al. Early insights into the role of Exoc6B associated with spondyloepimetaphyseal dysplasia with joint laxity type 3 in primary ciliogenesis and chondrogenic differentiation in vitro. Mol Biol Rep 51, 274 (2024). https://doi.org/10.1007/s11033-023-09114-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09114-9