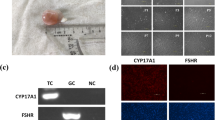
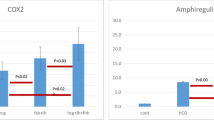
Abstract
Background
Devising of an appropriate in vitro culture method for germ cells differentiation in the presence of soluble factors has attracted considerable attention, which results will provide new insight into reproductive biology. In this study, we compared the effects of forskolin, retinoic acid (RA) or granulosa cell-conditioned medium in the presence or absence of granulosa cell co-culturing on germ cell differentiation from embryonic stem cells (ESCs).
Methods and Results
Embryonic stem cells were differentiated using embryoid bodies (EBs) for 5 days, and then EB-derived cells were co-cultured with or without adult mouse granulosa cells using monolayer protocol and treated with 50 µM forskolin, 1 µM RA and 50% granulosa cell-conditioned medium for 4 days. Granulosa cell-conditioned medium significantly increased the levels of Scp3, Rec8, Mvh and Gdf9 expression in the granulosa cell co-culture method compared to untreated cells. A significant elevation of Stra8, Rec8 and Mvh was observed after treatment with RA in the absence of granulosa cells and there was no significant increase in the levels of expression of germ cell-specific genes after treatment with forskolin compared to control. Furthermore, forskolin and RA significantly increased viability and proliferation of germ-like cells, compared with granulosa cell-conditioned medium.
Conclusions
Our study revealed that granulosa cell-conditioned medium and RA effectively can induce germ cell differentiation from ESCs, however combined application of granulosa cell-conditioned medium and co-culturing with granulosa cells had synergic effect on germ cell development in vitro as optimized protocol.





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Hong T-K, Song J-H, Lee S-B, Do J-T (2021) Germ Cell Derivation from Pluripotent Stem Cells for Understanding. Vitro Gametogenesis Cells volume 81889. https://doi.org/10.3390/cells10081889
Anderson RA, Fulton N, Cowan G, Coutts S, Saunders PTK (2007) Conserved and divergent patterns of expression of DAZL, VASA and OCT4 in the germ cells of the human fetal ovary and testis. BMC Dev Biology volume 1136. https://doi.org/10.1186/1471-213X-7-136
Schleif MC, Havel SL, Griswold MD (2022) Function of Retinoic Acid in Development of Male and Female Gametes. Nutrients volume(6):1293
Bhattacharya I, Sharma P, Purohit S, Kothiyal S, Das M, Banerjee A (2022) Recent Update on Retinoic Acid-Driven Initiation of Spermatogonial Differentiation.Frontiers in Cell and Developmental Biologyvolume:833759–833759
Qing T, Shi Y, Qin H, Ye X, Wei W, Liu H, Ding M et al (2007) Induction of oocyte-like cells from mouse embryonic stem cells by co-culture with ovarian granulosa cells. Differ volume 10902–911. https://doi.org/10.1111/j.1432-0436.2007.00181.x
Chen H-F, Jan P-S, Kuo H-C, Wu F-C, Lan C-W, Huang M-C, Chien C-L et al (2014) Granulosa cells and retinoic acid co-treatment enrich potential germ cells from manually selected Oct4-EGFP expressing human embryonic stem cells. Reproductive Biomed Online volume 3319–332. https://doi.org/10.1016/j.rbmo.2014.05.009
Ohta H, Kurimoto K, Okamoto I, Nakamura T, Yabuta Y, Miyauchi H, Yamamoto T et al (2017) In vitro expansion of mouse primordial germ cell-like cells recapitulates an epigenetic blank slate. EMBO J volume 131888–1907. https://doi.org/10.15252/embj.201695862
Jung D, Xiong J, Ye M, Qin X, Li L, Cheng S, Luo M et al (2017) In vitro differentiation of human embryonic stem cells into ovarian follicle-like cells. Nat Commun volume 11–13. https://doi.org/10.1038/ncomms15680
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M (2012) Offspring from oocytes derived from in vitro primordial germ cell–like cells in mice. Sci volume 6109971–975. https://doi.org/10.1126/science.1226889
Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M et al (2016) Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell volume 3330–340. https://doi.org/10.1016/j.stem.2016.01.017
Uyar A, Torrealday S, Seli E (2013) Cumulus and granulosa cell markers of oocyte and embryo quality. Fertility and Sterility volume 4979–997. https://doi.org/10.1016/j.fertnstert.2013.01.129
Bahmanpour S, Khozani TT, Jaberipour M, Hosseini A, Esmaeilpour T (2013) A comparison of the multiple oocyte maturation gene expression patterns between the newborn and adult mouse ovary.Iranian Journal of Reproductive Medicine volume(10):815
Shah SM, Saini N, Ashraf S, Singh MK, Manik RS, Singla SK, Palta P et al (2017) Cumulus cell-conditioned medium supports embryonic stem cell differentiation to germ cell-like cells. Reprod Fertility Dev volume 4679–693. https://doi.org/10.1071/RD15159
Kobayashi M, Kobayashi M, Odajima J, Shioda K, Hwang YS, Sasaki K, Chatterjee P et al (2022) Expanding homogeneous culture of human primordial germ cell-like cells maintaining germline features without serum or feeder layers.Stem Cell Reports volume(3):507–521
Bahmanpour S, Zarei Fard N, Talaei-Khozani T, Hosseini A, Esmaeilpour T (2015) Effect of BMP 4 preceded by retinoic acid and co‐culturing ovarian somatic cells on differentiation of mouse embryonic stem cells into oocyte‐like cells. Dev Growth Differ volume 5378–388. https://doi.org/10.1111/dgd.12217
Lim JJ, Shim MS, Lee JE, Lee DR (2014) Three-step method for proliferation and differentiation of human embryonic stem cell (hESC)-derived male germ cells. PloS one volume 4e90454. https://doi.org/10.1371/journal.pone.0090454
Soleimani A, Fard NZ, Talaei-Khozani T, Bahmanpour S (2020) Epidermal growth factor and three‐dimensional scaffolds provide conducive environment for differentiation of mouse embryonic stem cells into oocyte‐like cells. Cell Biology International volume 91850–1859. https://doi.org/10.1002/cbin.11391
Rhinn M, Dollé P (2012) Retinoic acid signalling during development.Development volume(5):843–858
Xuemei L, Jing Y, Bei X, Juan H, Xinling R, Qun L, Guijin Z (2013) Retinoic acid improve germ cell differentiation from human embryonic stem cells.Iranian Journal of Reproductive Medicine volume(11):905
Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, Dev A et al (2006) In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell volume 1125–132. https://doi.org/10.1016/j.devcel.2006.05.010
Silva C, Wood JR, Salvador L, Zhang Z, Kostetskii I, Williams CJ, Strauss JF III (2009) Expression profile of male germ cell-associated genes in mouse embryonic stem cell cultures treated with all‐trans retinoic acid and testosterone. Molecular Reproduction and Development: Incorporating Gamete Research volume(1):11–21
Sasabe M, Terada T, Tsutsumi Y (1990) A preliminary report on the effect of forskolin on the meiotic progress of germ cells into prophase I in fetal rabbit ovaries cultured in vitro.Journal of the Faculty of Applied Biological Science, Hiroshima University volume(2):87–94
Yao HH-C, Capel B (2002) Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biology volume 2356–365. https://doi.org/10.1006/dbio.2002.0663
Bahmanpour S, Keshavarz A, Fard NZ (2020) Effect of different concentrations of forskolin along with mature granulosa cell co-culturing on mouse embryonic stem cell differentiation into germ-like cells. Iran Biomedical J volume 130. https://doi.org/10.29252/ibj.24.1.30
Sakai Y, Nakamura T, Okamoto I, Gyobu-Motani S, Ohta H, Yabuta Y, Tsukiyama T et al (2020) Induction of the germ cell fate from pluripotent stem cells in cynomolgus monkeys. Biology of Reproduction volume 3620–638. https://doi.org/10.1093/biolre/ioz205
Zhao Y, Ye S, Liang D, Wang P, Fu J, Ma Q, Kong R et al (2018) In vitro modeling of human germ cell development using pluripotent stem cells. Stem Cell Reports volume 2509–523. https://doi.org/10.1016/j.stemcr.2018.01.001
Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC (1993) Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proceedings of the National Academy of Sciences volume(18):8424–8428. https://doi.org/10.1073/pnas.90.18.8424
Sèdes L, Leclerc A, Moindjie H, Cate RL, Picard J-Y, Di Clemente N, Jamin SP (2013) Anti-Müllerian hormone recruits BMPR-IA in immature granulosa cells. PLOS ONE volume 11e81551. https://doi.org/10.1371/journal.pone.0081551
Campbell KL (1979) Ovarian granulosa cells isolated with EGTA and hypertonic sucrose: cellular integrity and function.Biology of Reproduction volume(4):773–786
Bahmanpour S, Talaei Khozani T, Soleimani A, Zareifard N (2020) Germ cell differentiation of mouse embryonic stem cells can be influenced by the culture medium. Biotech Histochem volume 3210–218. https://doi.org/10.1080/10520295.2019.1665711
Zolfaghar M, Mirzaeian L, Beiki B, Naji T, Moini A, Eftekhari-Yazdi P, Akbarinejad V et al (2020) Wharton’s jelly derived mesenchymal stem cells differentiate into oocyte like cells in vitro by follicular fluid and cumulus cells conditioned medium. Heliyon volume 10e04992. https://doi.org/10.1016/j.heliyon.2020.e04992
Malekshah AK, Moghaddam AE, Daraka SM (2006) Comparison of conditioned medium and direct co-culture of human granulosa cells on mouse embryo development.Indian Journal of Experimental Biology volume(3):189–192
Vanderstichele H, Delaey B, De Winter J, De Jong F, Rombauts L, Verhoeven G, Dello C et al (1994) Secretion of steroids, growth factors, and cytokines by immortalized mouse granulosa cell lines. Biology of Reproduction volume 51190–1202. https://doi.org/10.1095/biolreprod50.5.1190
Yamauchi K, Hasegawa K, Chuma S, Nakatsuji N, Suemori H (2009) In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLOS ONE volume 4e5338. https://doi.org/10.1371/journal.pone.0005338
Qing T, Liu H, Wei W, Ye X, Shen W, Zhang D, Song Z et al (2008) Mature oocytes derived from purified mouse fetal germ cells. Hum Reprod volume 154–61. https://doi.org/10.1093/humrep/dem334
Chen W, Jia W, Wang K, Zhou Q, Leng Y, Duan T, Kang J (2012) Retinoic acid regulates germ cell differentiation in mouse embryonic stem cells through a Smad-dependent pathway. Biochem Biophys Res Commun volume 3571–577. https://doi.org/10.1016/j.bbrc.2012.01.078
Kipp JL, Golebiowski A, Rodriguez G, Demczuk M, Kilen SM, Mayo KE (2011) Gene expression profiling reveals Cyp26b1 to be an activin regulated gene involved in ovarian granulosa cell proliferation. Endocrinol volume 1303–312. https://doi.org/10.1210/en.2010-0749
Jørgensen A, Nielsen J, Perlman S, Lundvall L, Mitchell R, Juul A, Rajpert-De Meyts E (2015) Ex vivo culture of human fetal gonads: manipulation of meiosis signalling by retinoic acid treatment disrupts testis development. Hum Reprod volume 102351–2363. https://doi.org/10.1093/humrep/dev194
Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC (2004) Positional cloning and characterization of mouse mei8, a disrupted allele of the meiotic cohesin Rec8. genesis volume(3):184–194. https://doi.org/10.1002/gene.20085
Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC (2006) Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proceedings of the National Academy of Sciences volume(8):2474–2479. https://doi.org/10.1073/pnas.0510813103
Tedesco M, Desimio MG, Klinger FG, De Felici M, Farini D (2013) Minimal concentrations of retinoic acid induce stimulation by retinoic acid 8 and promote entry into meiosis in isolated pregonadal and gonadal mouse primordial germ cells. Biology of Reproduction volume 6145 141 – 111. https://doi.org/10.1095/biolreprod.112.106526
Wang Y, Teng Z, Li G, Mu X, Wang Z, Feng L, Niu W et al (2015) Cyclic AMP in oocytes controls meiotic prophase I and primordial folliculogenesis in the perinatal mouse ovary. Dev volume 2343–351. https://doi.org/10.1242/dev.112755
Farini D, Scaldaferri ML, Iona S, La Sala G, De Felici M (2005) Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Dev Biology volume 149–56. https://doi.org/10.1016/j.ydbio.2005.06.036
Richards M, Fong C-Y, Bongso A (2010) Comparative evaluation of different in vitro systems that stimulate germ cell differentiation in human embryonic stem cells. Fertility and Sterility volume 3986–994. https://doi.org/10.1016/j.fertnstert.2008.10.030
Aflatoonian B, Ruban L, Jones M, Aflatoonian R, Fazeli A, Moore H (2009) In vitro post-meiotic germ cell development from human embryonic stem cells. Hum Reprod volume 123150–3159. https://doi.org/10.1093/humrep/dep334
Losino N, Luzzani C, Solari C, Boffi J, Tisserand ML, Sevlever G, Baranao L et al(2010) Maintenance of murine embryonic stem cells’ self-renewal and pluripotency with increase in proliferation rate by a bovine granulosa cell line-conditioned medium. Stem Cells and Development volume 81439–1449. https://doi.org/10.1089/scd.2010.0336
Takabayashi S, Sasaoka Y, Yamashita M, Tokumoto T, Ishikawa K, Noguchi M (2001) Novel growth factor supporting survival of murine primordial germ cells: evidence from conditioned medium of ter fetal gonadal somatic cells. Mol Reprod Development: volume 3384–396. https://doi.org/10.1002/mrd.1101
Ruijtenberg S, van den Heuvel S (2016) Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle volume 2196–212. https://doi.org/10.1080/15384101.2015.1120925
Acknowledgements
The work was done at the laboratory for stem cells research in Anatomy Department of Shiraz University of Medical Sciences, Shiraz, Iran. The authors would like to thank Mrs Salmannezhad and Sani for their technical assistances.
Funding
The project was assigned as grant number 7912 in the office of the Vice Chancellor of Research Affairs, Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
Z.M. and N.Z. performed the experiments and was involved in the collection, analysis, and interpretation of data and manuscript drafting. S.B. and N.Z. conceived the original idea and supervised the project. In addition, S.B. and N.Z. interpreted the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahmanpour, S., Moasses, Z. & Zarei-Fard, N. Comparative effects of retinoic acid, granulosa cells conditioned medium or forskolin in combination with granulosa cell co-culturing on mouse germ cell differentiation. Mol Biol Rep 50, 631–640 (2023). https://doi.org/10.1007/s11033-022-07920-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07920-1