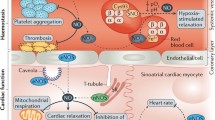
Abstract
Nitric oxide (NO) has essential roles in heart physiology, including the regulation of myocardial contractility and coronary blood flow, and in heart pathophysiology, particularly in the ischemic heart. NO is produced by both NO synthase (NOS)-dependent and NOS-independent pathways in the heart. This review summarizes quantitative aspects of NO production in the heart; the contribution of cardiomyocytes, endothelial cells (ECs), red blood cells (RBCs), and neurons are also discussed. Based on the available data, under normal conditions, the human heart produces about 50–70 µmol NO per day, primarily attributed to eNOS activity; ECs produce the highest amount of NO compared to other cell types in the heart. On the other hand, during ischemic conditions, NOS-independent NO production participates a significant role in the heart NO production that can exceed NOS-dependent NO generation. These data are relevant as most cardiovascular disorders are associated with NO dysfunction, and increasing NO bioavailability and signaling is a potential therapeutic approach for cardiovascular diseases.

Similar content being viewed by others
References
Liu VW, Huang PL (2008) Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovascular research 77 (1):19–29. https://doi.org/10.1016/j.cardiores.2007.06.024.
Vidanapathirana AK, Psaltis PJ, Bursill CA, Abell AD, Nicholls SJ (2021) Cardiovascular bioimaging of nitric oxide: Achievements, challenges, and the future. 41 (1):435–463. https://doi.org/10.1002/med.21736.
Ghasemi A (2022) Quantitative aspects of nitric oxide production from nitrate and nitrite. EXCLI Journal 21:470–486. https://doi.org/10.17179/excli2022-4727.
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33 (7):829–837, 837a-837d. https://doi.org/10.1093/eurheartj/ehr304.
Hare JM (2004) Spatial confinement of isoforms of cardiac nitric-oxide synthase: unravelling the complexities of nitric oxide’s cardiobiology. Lancet (London, England) 363 (9418):1338–1339. https://doi.org/10.1016/s0140-6736(04)16083-2.
Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416 (6878):337–339. https://doi.org/10.1038/416337a.
Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM (2009) Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation 119 (20):2656–2662. https://doi.org/10.1161/circulationaha.108.822205.
Giraldez RR, Panda A, Xia Y, Sanders SP, Zweier JL (1997) Decreased nitric-oxide synthase activity causes impaired endothelium-dependent relaxation in the postischemic heart. The Journal of biological chemistry 272 (34):21420–21426. https://doi.org/10.1074/jbc.272.34.21420.
Samouilov A, Kuppusamy P, Zweier JL (1998) Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Archives of Biochemistry and Biophysics 357 (1):1–7. https://doi.org/10.1006/abbi.1998.0785.
Bloch KD, Janssens S (2005) Cardiomyocyte-specific overexpression of nitric oxide synthase 3: impact on left ventricular function and myocardial infarction. Trends in Cardiovascular Medicine 15 (7):249–253. https://doi.org/10.1016/j.tcm.2005.07.005.
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD (2016) Revisiting Cardiac Cellular Composition. Circulation research 118 (3):400–409. https://doi.org/10.1161/circresaha.115.307778.
Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J (2015) Dynamics of Cell Generation and Turnover in the Human Heart. Cell 161 (7):1566–1575. https://doi.org/10.1016/j.cell.2015.05.026.
Wang Y, Ma X (2021) Healthy Coronary Endothelial Cells, Happy Cardiomyocytes. Circulation 143 (6):581–582. https://doi.org/10.1161/circulationaha.120.052535.
Buchwalow IB, Schulze W, Karczewski P, Kostic MM, Wallukat G, Morwinski R, Krause EG, Müller J, Paul M, Slezak J, Luft FC, Haller H (2001) Inducible nitric oxide synthase in the myocard. Molecular and cellular biochemistry 217 (1–2):73–82. https://doi.org/10.1023/a:1007286602865.
Ursell PC, Mayes M (1993) The majority of nitric oxide synthase in pig heart is vascular and not neural. Cardiovascular research 27 (11):1920–1924. https://doi.org/10.1093/cvr/27.11.1920.
Kelm M, Schrader J (1988) Nitric oxide release from the isolated guinea pig heart. European journal of pharmacology 155 (3):317–321. https://doi.org/10.1016/0014-2999(88)90522-5.
Tsukada Y, Yasutake M, Jia D, Kusama Y, Kishida H, Takano T, Tsukada S (2003) Real-time measurement of nitric oxide by luminol-hydrogen peroxide reaction in crystalloid perfused rat heart. Life sciences 72 (9):989–1000. https://doi.org/10.1016/s0024-3205(02)02353-6.
Hattler BG, Oddis CV, Zeevi A, Luss H, Shah N, Geller DA, Billiar TR, Simmons RL, Finkel MS (1995) Regulation of constitutive nitric oxide synthase activity by the human heart. The American journal of cardiology 76 (12):957–959. https://doi.org/10.1016/s0002-9149(99)80269-0.
Pauziene N, Rysevaite-Kyguoliene K, Alaburda P, Pauza AG, Skukauskaite M, Masaityte A, Laucaityte G, Saburkina I, Inokaitis H, Plisiene J, Pauza DH (2017) Neuroanatomy of the Pig Cardiac Ventricles. A Stereomicroscopic, Confocal and Electron Microscope Study. 300 (10):1756–1780. https://doi.org/10.1002/ar.23619.
Kelm M, Schrader J (1990) Control of coronary vascular tone by nitric oxide. Circulation research 66 (6):1561–1575. https://doi.org/10.1161/01.res.66.6.1561.
Suárez J, Torres C, Sánchez L, del Valle L, Pastelín G (1999) Flow stimulates nitric oxide release in guinea pig heart: role of stretch-activated ion channels. Biochemical and biophysical research communications 261 (1):6–9. https://doi.org/10.1006/bbrc.1999.1005.
Van Liere EJ, Sleeth. CK (1936) Normal heart-weight, body-weight (HW/BW) ratio in the guinea pig. Proceedings of the Society for Experimental Biology and Medicine 34 (1):41–41. https://doi.org/10.1258/acb.2012.012168.
Snyder W, Cook M, Nasset E, Karhausen L, Tipton I (1975) Report of the task group on reference man. Report Prepared for International Commission on Radiological Protection, vol 23. Oxford. doi:10.1016/s0074-2740(75)80015-8
Zweier JL, Wang P, Samouilov A, Kuppusamy P (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nature medicine 1 (8):804–809. https://doi.org/10.1038/nm0895-804.
Morishita T, Tsutsui M, Shimokawa H, Sabanai K, Tasaki H, Suda O, Nakata S, Tanimoto A, Wang KY, Ueta Y, Sasaguri Y, Nakashima Y, Yanagihara N (2005) Nephrogenic diabetes insipidus in mice lacking all nitric oxide synthase isoforms. Proceedings of the National Academy of Sciences of the United States of America 102 (30):10616–10621. https://doi.org/10.1073/pnas.0502236102.
Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet (London, England) 363 (9418):1365–1367. https://doi.org/10.1016/s0140-6736(04)16048-0.
Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M (2003) Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35 (7):790–796. https://doi.org/10.1016/s0891-5849(03)00406-4.
Omar SA, Webb AJ (2014) Nitrite reduction and cardiovascular protection. Journal of molecular and cellular cardiology 73:57–69. https://doi.org/10.1016/j.yjmcc.2014.01.012.
Bryan NS, Calvert JW, Gundewar S, Lefer DJ (2008) Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med 45 (4):468–474. https://doi.org/10.1016/j.freeradbiomed.2008.04.040.
Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ (2007) Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America 104 (48):19144–19149. https://doi.org/10.1073/pnas.0706579104.
Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M (2005) Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1 (5):290–297. https://doi.org/10.1038/nchembio734.
Almeida LE, Kamimura S, Kenyon N, Khaibullina A, Wang L, de Souza Batista CM, Quezado ZM (2015) Validation of a method to directly and specifically measure nitrite in biological matrices. Nitric oxide : biology and chemistry 45:54–64. https://doi.org/10.1016/j.niox.2014.10.008.
Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, Ong HL, Adams D, Gahl WA, Zheng C, Qi S, Jin L, Zhang C, Gu L, He J, Deng D, Ambudkar IS, Wang S (2012) Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America 109 (33):13434–13439. https://doi.org/10.1073/pnas.1116633109.
Reimer RJ (2013) SLC17: a functionally diverse family of organic anion transporters. Molecular aspects of medicine 34 (2–3):350–359. https://doi.org/10.1016/j.mam.2012.05.004.
Verheijen FW, Verbeek E, Aula N, Beerens CE, Havelaar AC, Joosse M, Peltonen L, Aula P, Galjaard H, van der Spek PJ, Mancini GM (1999) A new gene, encoding an anion transporter, is mutated in sialic acid storage diseases. Nature genetics 23 (4):462–465. https://doi.org/10.1038/70585.
Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7 (2):156–167. https://doi.org/10.1038/nrd2466.
Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen GY, Takematsu H, Kozutsumi Y, Suzuki A, Furuhata K, Cheng FL, Lin CH, Sato C, Kitajima K, Kannagi R (2006) Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer research 66 (6):2937–2945. https://doi.org/10.1158/0008-5472.can-05-2615.
Conte E, Fonzino A, Cibelli A, De Benedictis V, Imbrici P, Nicchia GP, Pierno S, Camerino GM (2020) Changes in Expression and Cellular Localization of Rat Skeletal Muscle ClC-1 Chloride Channel in Relation to Age, Myofiber Phenotype and PKC Modulation. Frontiers in pharmacology 11:714. https://doi.org/10.3389/fphar.2020.00714.
Srihirun S, Park JW, Teng R, Sawaengdee W, Piknova B, Schechter AN (2020) Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric oxide : biology and chemistry 94:1–8. https://doi.org/10.1016/j.niox.2019.10.005.
Kapil V, Khambata R, Jones D, Rathod K, Primus C, Massimo G, Fukuto J, Ahluwalia A (2020) The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacological Reviews 72 (3):692–766. https://doi.org/10.1124/pr.120.019240.
Calvert JW, Lefer DJ (2010) Clinical translation of nitrite therapy for cardiovascular diseases. Nitric oxide : biology and chemistry 22 (2):91–97. https://doi.org/10.1016/j.niox.2009.11.001.
Eriksson KE, Yang T, Carlström M, Weitzberg E (2018) Organ uptake and release of inorganic nitrate and nitrite in the pig. Nitric oxide : biology and chemistry 75:16–26. https://doi.org/10.1016/j.niox.2018.02.001.
Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M (2004) Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proceedings of the National Academy of Sciences of the United States of America 101 (12):4308–4313. https://doi.org/10.1073/pnas.0306706101.
Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO (2008) A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nature Chemical Biology 4 (7):411–417. https://doi.org/10.1038/nchembio.92.
Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL (2008) Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. The Journal of biological chemistry 283 (26):17855–17863. https://doi.org/10.1074/jbc.M801785200.
Zweier JL, Li H, Samouilov A, Liu X (2010) Mechanisms of nitrite reduction to nitric oxide in the heart and vessel wall. Nitric oxide : biology and chemistry 22 (2):83–90. https://doi.org/10.1016/j.niox.2009.12.004.
Li H, Samouilov A, Liu X, Zweier JL (2003) Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 42 (4):1150–1159. https://doi.org/10.1021/bi026385a.
van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT (2009) Nitrite as regulator of hypoxic signaling in mammalian physiology. Medicinal research reviews 29 (5):683–741. https://doi.org/10.1002/med.20151.
Li H, Samouilov A, Liu X, Zweier JL (2001) Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. The Journal of biological chemistry 276 (27):24482–24489. https://doi.org/10.1074/jbc.M011648200.
Milsom AB, Fernandez BO, Garcia-Saura MF, Rodriguez J, Feelisch M (2012) Contributions of nitric oxide synthases, dietary nitrite/nitrate, and other sources to the formation of NO signaling products. Antioxidants & redox signaling 17 (3):422–432. https://doi.org/10.1089/ars.2011.4156.
Gayeski TE, Honig CR (1991) Intracellular PO2 in individual cardiac myocytes in dogs, cats, rabbits, ferrets, and rats. The American journal of physiology 260 (2 Pt 2):H522-531. https://doi.org/10.1152/ajpheart.1991.260.2.H522.
Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL (2005) Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation 111 (22):2966–2972. https://doi.org/10.1161/circulationaha.104.527226.
Trochu JN, Bouhour JB, Kaley G, Hintze TH (2000) Role of endothelium-derived nitric oxide in the regulation of cardiac oxygen metabolism: implications in health and disease. Circulation research 87 (12):1108–1117. https://doi.org/10.1161/01.res.87.12.1108.
Al-Obaidi MK, Etherington PJ, Barron DJ, Winlove CP, Pepper JR (2000) Myocardial tissue oxygen supply and utilization during coronary artery bypass surgery: evidence of microvascular no-reflow. Clinical science (London, England : 1979) 98 (3):321–328.
Zweier JL, Samouilov A, Kuppusamy P (1999) Non-enzymatic nitric oxide synthesis in biological systems. Biochimica et biophysica acta 1411 (2–3):250–262. https://doi.org/10.1016/s0005-2728(99)00018-3.
Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A (2004) Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proceedings of the National Academy of Sciences of the United States of America 101 (37):13683–13688. https://doi.org/10.1073/pnas.0402927101.
Duranski MR, Greer JJM, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet S-F, Wang X, Kevil CG, Gladwin MT, Lefer DJ (2005) Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115 (5):1232–1240. https://doi.org/10.1172/JCI22493.
Balligand JL, Kobzik L, Han X, Kaye DM, Belhassen L, O’Hara DS, Kelly RA, Smith TW, Michel T (1995) Nitric oxide-dependent parasympathetic signaling is due to activation of constitutive endothelial (type III) nitric oxide synthase in cardiac myocytes. The Journal of biological chemistry 270 (24):14582–14586. https://doi.org/10.1074/jbc.270.24.14582.
Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T (1996) Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. The Journal of biological chemistry 271 (37):22810–22814. https://doi.org/10.1074/jbc.271.37.22810.
Wei C, Jiang S, Lust JA, Daly RC, McGregor CG (1996) Genetic expression of endothelial nitric oxide synthase in human atrial myocardium. Mayo Clinic proceedings 71 (4):346–350. https://doi.org/10.4065/71.4.346.
Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC (1999) Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America 96 (2):657–662. https://doi.org/10.1073/pnas.96.2.657.
Haywood GA, Tsao PS, von der Leyen HE, Mann MJ, Keeling PJ, Trindade PT, Lewis NP, Byrne CD, Rickenbacher PR, Bishopric NH, Cooke JP, McKenna WJ, Fowler MB (1996) Expression of inducible nitric oxide synthase in human heart failure. Circulation 93 (6):1087–1094. https://doi.org/10.1161/01.cir.93.6.1087.
Patten RD, Denofrio D, El-Zaru M, Kakkar R, Saunders J, Celestin F, Warner K, Rastegar H, Khabbaz KR, Udelson JE, Konstam MA, Karas RH (2005) Ventricular assist device therapy normalizes inducible nitric oxide synthase expression and reduces cardiomyocyte apoptosis in the failing human heart. Journal of the American College of Cardiology 45 (9):1419–1424. https://doi.org/10.1016/j.jacc.2004.05.090.
de Belder AJ, Radomski MW, Why HJ, Richardson PJ, Bucknall CA, Salas E, Martin JF, Moncada S (1993) Nitric oxide synthase activities in human myocardium. Lancet (London, England) 341 (8837):84–85. https://doi.org/10.1016/0140-6736(93)92559-c.
Balligand JL, Kelly RA, Marsden PA, Smith TW, Michel T (1993) Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proceedings of the National Academy of Sciences of the United States of America 90 (1):347–351. https://doi.org/10.1073/pnas.90.1.347.
Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kühn B (2013) Cardiomyocyte proliferation contributes to heart growth in young humans. Proceedings of the National Academy of Sciences of the United States of America 110 (4):1446–1451. https://doi.org/10.1073/pnas.1214608110.
Siervo M, Stephan BC, Feelisch M, Bluck LJ (2011) Measurement of in vivo nitric oxide synthesis in humans using stable isotopic methods: a systematic review. Free Radic Biol Med 51 (4):795–804. https://doi.org/10.1016/j.freeradbiomed.2011.05.032.
Bachetti T, Comini L, Curello S, Bastianon D, Palmieri M, Bresciani G, Callea F, Ferrari R (2004) Co-expression and modulation of neuronal and endothelial nitric oxide synthase in human endothelial cells. Journal of molecular and cellular cardiology 37 (5):939–945. https://doi.org/10.1016/j.yjmcc.2004.07.006.
Lührs H, Papadopoulos T, Schmidt HH, Menzel T (2002) Type I nitric oxide synthase in the human lung is predominantly expressed in capillary endothelial cells. Respiration physiology 129 (3):367–374. https://doi.org/10.1016/s0034-5687(01)00323-1.
Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F, Piragine E, Schneckmann R, Hutzler B, Good ME, Fernandez BO, Vornholz L, Rogers S, Doctor A, Grandoch M, Stegbauer J, Weitzberg E, Feelisch M, Lundberg JO, Isakson BE, Kelm M, Cortese-Krott MM (2021) Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 144 (11):870–889. https://doi.org/10.1161/circulationaha.120.049606.
Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN (2005) Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood 106 (2):734–739. https://doi.org/10.1182/blood-2005-02-0567.
Chen K, Popel AS (2006) Theoretical analysis of biochemical pathways of nitric oxide release from vascular endothelial cells. Free Radic Biol Med 41 (4):668–680. https://doi.org/10.1016/j.freeradbiomed.2006.05.009.
Kelm M, Feelisch M, Deussen A, Strauer BE, Schrader J (1991) Release of endothelium derived nitric oxide in relation to pressure and flow. Cardiovascular research 25 (10):831–836. https://doi.org/10.1093/cvr/25.10.831.
Kelm M, Feelisch M, Spahr R, Piper HM, Noack E, Schrader J (1988) Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochemical and biophysical research communications 154 (1):236–244. https://doi.org/10.1016/0006-291x(88)90675-4.
Sender R, Fuchs S, Milo R (2016) Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 14 (8):e1002533. https://doi.org/10.1371/journal.pbio.1002533.
Arnal JF, Clamens S, Pechet C, Negre-Salvayre A, Allera C, Girolami JP, Salvayre R, Bayard F (1996) Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proceedings of the National Academy of Sciences of the United States of America 93 (9):4108–4113. https://doi.org/10.1073/pnas.93.9.4108.
Kuchan MJ, Frangos JA (1994) Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. The American journal of physiology 266 (3 Pt 1):C628-636. https://doi.org/10.1152/ajpcell.1994.266.3.C628.
Hood JD, Meininger CJ, Ziche M, Granger HJ (1998) VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. The American journal of physiology 274 (3):H1054-1058. https://doi.org/10.1152/ajpheart.1998.274.3.H1054.
Brovkovych V, Stolarczyk E, Oman J, Tomboulian P, Malinski T (1999) Direct electrochemical measurement of nitric oxide in vascular endothelium. Journal of pharmaceutical and biomedical analysis 19 (1–2):135–143. https://doi.org/10.1016/s0731-7085(98)00090-9.
Haas TL, Duling BR (1997) Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvascular research 53 (2):113–120. https://doi.org/10.1006/mvre.1996.1999.
Cortese-Krott MM, Rodriguez-Mateos A, Sansone R, Kuhnle GG, Thasian-Sivarajah S, Krenz T, Horn P, Krisp C, Wolters D, Heiss C, Kroncke KD, Hogg N, Feelisch M, Kelm M (2012) Human red blood cells at work: identification and visualization of erythrocytic eNOS activity in health and disease. Blood 120 (20):4229–4237. https://doi.org/10.1182/blood-2012-07-442277.
Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA (1997) Gross and microscopic anatomy of the human intrinsic cardiac nervous system. The Anatomical record 247 (2):289–298. https://doi.org/10.1002/(sici)1097-0185(199702)247:2<289::aid-ar15>3.0.co;2-l.
Kawano H, Okada R, Yano K (2003) Histological study on the distribution of autonomic nerves in the human heart. Heart and vessels 18 (1):32–39. https://doi.org/10.1007/s003800300005.
Klimaschewski L, Kummer W, Mayer B, Couraud JY, Preissler U, Philippin B, Heym C (1992) Nitric oxide synthase in cardiac nerve fibers and neurons of rat and guinea pig heart. Circulation research 71 (6):1533–1537. https://doi.org/10.1161/01.res.71.6.1533.
Sosunov AA, Hassall CJ, Loesch A, Turmaine M, Burnstock G (1996) Nitric oxide synthase-containing neurones and nerve fibres within cardiac ganglia of rat and guinea-pig: an electron-microscopic immunocytochemical study. Cell and tissue research 284 (1):19–28. https://doi.org/10.1007/s004410050563.
Navickaite I, Pauziene N, Pauza DH (2021) Anatomical evidence of non-parasympathetic cardiac nitrergic nerve fibres in rat. Journal of anatomy 238 (1):20–35. https://doi.org/10.1111/joa.13291.
Park CS, Park R, Krishna G (1996) Constitutive expression and structural diversity of inducible isoform of nitric oxide synthase in human tissues. Life sciences 59 (3):219–225. https://doi.org/10.1016/0024-3205(96)00287-1.
Gath I, Closs EI, Gödtel-Armbrust U, Schmitt S, Nakane M, Wessler I, Förstermann U (1996) Inducible NO synthase II and neuronal NO synthase I are constitutively expressed in different structures of guinea pig skeletal muscle: implications for contractile function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 10 (14):1614–1620. https://doi.org/10.1096/fasebj.10.14.9002553.
Lundberg JO, Gladwin MT, Weitzberg E (2015) Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14 (9):623–641. https://doi.org/10.1038/nrd4623.
Funding
This study was supported by a grant (Grant No. 43002799-1) from Shahid Beheshti University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception. The first draft of the manuscript was written by Asghar Ghasemi, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Asghar Ghasemi declares that he has no conflict of interest. Sajad Jeddi declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghasemi, A., Jeddi, S. Quantitative aspects of nitric oxide production in the heart. Mol Biol Rep 49, 11113–11122 (2022). https://doi.org/10.1007/s11033-022-07889-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07889-x