Abstract
Background
Many chemotherapeutic drugs used in cancer treatment have anticancer properties by inducing reactive oxygen species (ROS). However, the same effect occurs in normal cells, limiting the availability of these drugs. Therefore, studies on the detection of new herbal anticancer agents that have selective effects on cancer cells are of great importance. The aim of this study is to investigate the metabolite profile of Cedrus libani tar and its mechanism of anticancer effect on colon cancer cells.
Methods and results
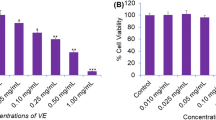
Effect of cedar tar on cells (12 cancers and 5 normal cell lines) viability was determined by MTT, apoptosis induction was determined by Annexin-V, ROS and MMP determined by flow cytometry assay. Cleaved caspase-8, 9 and Ɣ-H2AX expression determined by western blot. Apoptotic and antioxidant genes expression level determined by qPCR. Metabolite profiling was performed with LC–MS/MS and GC–MS. Cedar tar showed the highest cytotoxic effect among cancer cells in colon cancer (HCT-116, IC50: 30.4 μg/mL) and its toxic effect on normal cells (HUVEC, IC50: 74.07 μg/mL) was less than cancer cell. Cedar tar increases ROS production in colon cancer cells. The metabolite profile of the cedar tar contains high amounts of metabolites such as fatty acids mainly (Duprezianene, Himachalene and Chamigrene), phenolic compounds (mostly Coumarin, p-coumaric acid, Vanillic acid and tr-Ferulic acid etc.) and organic acids (mainly 3-oh propanoic acid, 2-oh butyric acid and 3-oh isovaleric acid etc.).
Conclusion
As a result, it has been found that cedar tar has the potential to be used in the treatment of colon cancer.
Graphical abstract






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [IK.], upon reasonable request.
Code availability
Not applicable.
References
Tunstall-Pedoe H (2005) Preventing chronic diseases. a vital investment: WHO global report. World Health Organization. Oxford University Press, Geneva
Xu J, Murphy SL, Kochanek KD, Arias E (2021) Deaths: final data for 2019. national vital statistics reports. https://www.cdc.gov/nchs/nvss/index.htm. Accessed 7 May 2021
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Kassebaum NJ, Smith AG, Bernabé E et al (2017) Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res 96:380–387. https://doi.org/10.1177/0022034517693566
Kilickap S, Akgul E, Aksoy S, Aytemir K, Barista I (2005) Doxorubicin-induced second degree and complete atrioventricular block. Europace 7:227–230. https://doi.org/10.1016/j.eupc.2004.12.012
Shiga T, Hiraide M (2020) Cardiotoxicities of 5-fluorouracil and other fluoropyrimidines. Curr Treat Option Oncol 21:1–21. https://doi.org/10.1007/s11864-020-0719-1
Sreenivasan J, Hooda U, Ranjan P, Jain D (2021) Nuclear imaging for the assessment of cardiotoxicity from chemotherapeutic agents in oncologic disease. Curr Cardiol Rep 23:1–9. https://doi.org/10.1007/s11886-021-01493-4
Desai AG, Qazi GN, Ganju RK et al (2008) Medicinal plants and cancer chemoprevention. Curr Drug Metab 9:581–591. https://doi.org/10.2174/138920008785821657
Dongre U, Meshram T, Dighe S, Narnawre K, Mehere B, Somkuwar SR (2020) Screening of selected ethno-medicinal plants for anti-cancer activity. Adv Zool Bot 8(5):447–452
Greenwell M, Rahman P (2015) Medicinal plants: their use in anticancer treatment. Int J Pharm Sci Res 6:4103–4112
Roy A, Bharadvaja N (2017) Medicinal plants in the management of cancer: a review. Int J Complement Alt Med 9:291–304. https://doi.org/10.1155/2020/3529081
Taneja S, Qazi G (2007) Bioactive molecues in medicinal plants: a perspective in their therapeutic action. Wiley Online Library.
Huang M, Lu J-J, Ding J (2021) Natural products in cancer therapy: past, present and future. Nat Prod Bioprospect 11:5–13. https://doi.org/10.1007/s13659-020-00293-7
Koyuncu I, Gönel A, Temiz E, Karaoğul E, Uyar Z (2021) Pistachio green hull extract induces apoptosis through multiple signaling pathways by causing oxidative stress on colon cancer cells. Anticancer Agents Med Chem 21:725–737. https://doi.org/10.2174/1871520620999200730155524
Park S-H, Kim M, Lee S, Jung W, Kim B (2021) Therapeutic potential of natural products in treatment of cervical cancer: a review. Nutrients 13:154. https://doi.org/10.3390/nu13010154
Akrep M (2013) Eskiçağ Anadolusu’nda Sedir Ağacının Önemi. Mavi Atla 1:21–27
Khuri S, Shmoury M, Baalbaki R, Maunder M, Talhouk S (2000) Conservation of the Cedrus libani populations in Lebanon: history, current status and experimental application of somatic embryogenesis. Biodivers Conserv 9:1261–1273. https://doi.org/10.1023/A:1008936104581
Bhattarai N (1992) Medical ethnobotany in the Karnali zone Nepal. Econ Bot 46(3):257–261
Kurt Y, Isik K (2012) Comparison of tar produced by traditional and laboratory methods. Ethno Med 6:77–83. https://doi.org/10.1080/09735070.2012.11886423
Kurt Y, Kaçar MS, Isik K (2008) Traditional tar production from Cedrus libani a. rich on the taurus mountains in southern turkey. Econ Bot 62:615–628. https://doi.org/10.1007/s12231-008-9023-x
Cetin H, Kurt Y, Isik K, Yanikoglu A (2009) Larvicidal effect of Cedrus libani seed oils on mosquito Culex pipiens. Pharm Biol 47:665–668. https://doi.org/10.1080/13880200902918360
Dığrak M, İlçim A, Hakkı Alma M (1999) Antimicrobial activities of several parts of Pinus brutia, Juniperus oxycedrus, Abies cilicia, Cedrus libani and Pinus nigra. Phytother Res 13:584–587. https://doi.org/10.1002/(SICI)1099-1573(199911)13:7
Kizil M, Kizil G, Yavuz M, Aytekin C (2002) Antimicrobial activity of the resins obtained from the roots and stems of Cedrus libani and Abies cilicica. Prikl Biokhim Mikrobiol 38:166–168. https://doi.org/10.1023/A:1014358532581
Yeşilada E, Il G, Shibata H (1999) Screening of Turkish anti-ulcerogenic folk remedies for anti-Helicobacter pylori activity. J Ethnopharmacol 66:289–293. https://doi.org/10.1016/S0378-8741(98)00219-0
Desai A, Gupta R, Advani S, Ouellette L, Kuderer NM, Lyman GH, Li A (2021) Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta-analysis of cohort studies. Cancer 127:1459–1468. https://doi.org/10.1002/cncr.33386
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Fulda S (2008) Betulinic acid for cancer treatment and prevention. Int J Mol Sci 9:1096–1107. https://doi.org/10.3390/ijms9061096
Pilatova M, Varinska L, Perjesi P et al (2010) In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicol In Vitro 24:1347–1355. https://doi.org/10.1016/j.tiv.2010.04.013
Gupta A, Naraniwal M, Kothari V (2012) Modern extraction methods for preparation of bioactive plant extracts. Int J Appl Nat Sci 1(1):8–26
Korkina L, Kostyuk V (2012) Biotechnologically produced secondary plant metabolites for cancer treatment and prevention. Curr Pharm Biotechnol 13:265–275. https://doi.org/10.2174/138920112798868692
Koyuncu I (2018) Evaluation of anticancer, antioxidant activity and phenolic compounds of Artemisia absinthium L extract. Cell Mol Biol 64(3):25–34
Loizzo MR, Saab A, Tundis R et al (2008) Phytochemical analysis and in vitro evaluation of the biological activity against herpes simplex virus type 1 (HSV-1) of Cedrus libani A Rich. Phytomedicine 15:79–83. https://doi.org/10.1016/j.phymed.2007.03.013
Loizzo MR, Tundis R, Conforti F, Saab AM, Statti GA, Menichini F (2007) Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chem 105:572–578. https://doi.org/10.1016/j.foodchem.2007.04.015
Saab AM, Gambari R, Sacchetti G et al (2018) Phytochemical and pharmacological properties of essential oils from Cedrus species. Nat Prod Res 32:1415–1427. https://doi.org/10.1080/14786419.2017.1346648
Saab AM, Lampronti I, Borgatti M, Finotti A, Harb F, Safi S, Gambari R (2012) In vitro evaluation of the anti-proliferative activities of the wood essential oils of three Cedrus species against K562 human chronic myelogenous leukaemia cells. Nat Prod Res 26:2227–2231. https://doi.org/10.1080/14786419.2011.643885
Saab AM, Lampronti I, Grandini A et al (2011) Antiproliferative and erythroid differentiation activities of Cedrus libani seed extracts against K562 human chronic myelogenus leukemia cells. Int J Pharm Biol Arch 2:1744–1748
Venditti A, Maggi F, Saab A et al (2020) Antiproliferative, antimicrobial and antioxidant properties of Cedrus libani and Pinus pinea wood oils and Juniperus excelsa berry oil. Plant Biosyst. https://doi.org/10.1080/11263504.2020.1864495
Chaudhary AK, Ahmad S, Mazumder A (2015) Isolation, structural elucidation and in vitro antioxidant activity of compounds from chloroform extract of Cedrus deodara (Roxb.) Loud. Nat Prod Res 29:268–273. https://doi.org/10.1080/14786419.2014.940946
Sharma S, Bhatt V, Kumar N, Singh B, Sharma U (2017) Locational comparison of essential oils from selected conifers of Himachal Pradesh. Nat Prod Res 31:1578–1582. https://doi.org/10.1080/14786419.2016.1277354
Erel SB, Demir S, Nalbantsoy A, Ballar P, Khan S, Yavasoglu NUK, Karaalp C (2014) Bioactivity screening of five Centaurea species and in vivo anti-inflammatory activity of C.athoa. Pharm Biol 52:775–781. https://doi.org/10.3109/13880209.2013.868493
Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H (2014) Evaluating medicinal plants for anticancer activity. Sci World J 2014:1–12. https://doi.org/10.1155/2014/721402
Koyuncu I, Gönel A, Akdağ A, Yilmaz MA (2018) Identification of phenolic compounds, antioxidant activity and anti-cancer effects of the extract obtained from the shoots of Ornithogalum narbonense L. Cell Mol Biol 64(1):75–83
Koyuncu I, Gonel A, Durgun M, Kocyigit A, Yuksekdag O, Supuran CT (2019) Assessment of the antiproliferative and apoptotic roles of sulfonamide carbonic anhydrase IX inhibitors in HeLa cancer cell line. J Enzyme Inhib Med Chem 34:75–86. https://doi.org/10.1080/14756366.2018.1524380
Obiorah IE, Fan P, Sengupta S, Jordan VC (2014) Selective estrogen-induced apoptosis in breast cancer. Steroids 90:60–70. https://doi.org/10.1016/j.steroids.2014.06.003
Temiz E, Koyuncu I, Saadat S, Yüksekdag O (2021) Exploring the antiproliferative mechanisms of urtica dioica L. extract in human promyelocytic leukemia cell line. J Harran Univ Med Fac 18:468–474
Bao H, Zhang Q, Zhu Z et al (2017) BHX, a novel pyrazoline derivative, inhibits breast cancer cell invasion by reversing the epithelial-mesenchymal transition and down-regulating Wnt/β-catenin signalling. Sci Rep 7:9153. https://doi.org/10.1038/s41598-017-09655-7
Liu Y, Zhu X (2017) Endoplasmic reticulum–mitochondria tethering in neurodegenerative diseases. Transl Neurodegener 6:21–30. https://doi.org/10.1186/s40035-017-0092-6
Villa-Pulgarín JA, Gajate C, Botet J et al (2017) Mitochondria and lipid raft-located FOF1-ATP synthase as major therapeutic targets in the antileishmanial and anticancer activities of ether lipid edelfosine. PLoS Negl Trop Dis 11:58–75. https://doi.org/10.1371/journal.pntd.0005805
Liu H, Xie J, Fan L, Xia Y, Peng X, Zhou J, Ni X (2022) Cryptotanshinone protects against PCOS-induced damage of ovarian tissue via regulating oxidative stress, mitochondrial membrane potential, inflammation, and apoptosis via regulating ferroptosis. Oxid Med Cell Longev 2022:1–21. https://doi.org/10.1155/2022/8011850
Yao J, Jiao R, Liu C, Zhang Y, Yu W, Lu Y, Tan R (2016) Assessment of the cytotoxic and apoptotic effects of chaetominine in a human leukemia cell line. Biomol Ther 24:147–155. https://doi.org/10.4062/biomolther.2015.093
Dadsena S, King LE, García-Sáez AJ (2021) Apoptosis regulation at the mitochondria membrane level. Biochim Biophys Acta 1863:183–193. https://doi.org/10.1016/j.bbamem.2021.183716
Bargonetti J, Manfredi JJ (2002) Multiple roles of the tumor suppressor p53. Curr Opin Oncol 14:86–91. https://doi.org/10.1097/00001622-200201000-00015
Kuczler MD, Olseen AM, Pienta KJ, Amend SR (2021) ROS-induced cell cycle arrest as a mechanism of resistance in polyaneuploid cancer cells (PACCs). Prog Biophys Mol Biol 165:3–7. https://doi.org/10.1016/j.pbiomolbio.2021.05.002
Bertoli C, Skotheim JM, De Bruin RA (2013) Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14:518–530. https://doi.org/10.1038/nrm3629
Murad H, Hawat M, Ekhtiar A et al (2016) Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int 16:39–52. https://doi.org/10.1186/s12935-016-0315-4
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
ET, IK, KE, and OY, conducted the experiments and contriubuted to data interpretation; ET, MT, OY, SA and YK participated in data analysis, discussion and manuscript preparation and all were involved in manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Temiz, E., Eği, K., Koyuncu, I. et al. Cedrus libani tar prompts reactive oxygen species toxicity and DNA damage in colon cancer cells. Mol Biol Rep 49, 7939–7952 (2022). https://doi.org/10.1007/s11033-022-07631-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07631-7