Abstract
Background
Calorie restriction (CR) is a type of dietary intervention that is essential in weight loss through modulation of critical metabolic control pathways, is well established and understood in cases of systemic arterial hypertension, however, its role in renovascular hypertension is still unclear.
Methods
Rats were divided into three groups: SHAM, and two groups that underwent surgery to clip the left renal artery and induce renovascular hypertension (OH and OHR). The SHAM diet was as follows: 14 weeks normolipidic diet; OH: 2 weeks normolipidic diet + 12 weeks hyperlipidic diet, both ad libitum; OHR, 2 weeks normolipidic diet + 8 weeks ad libitum high-fat diet + 4 weeks 40% calorie-restricted high-fat diet.
Results
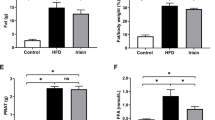
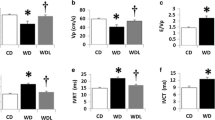
Rats in the OHR group had decreased blood pressure, body weight, and glucose levels. Reductions in insulinemia and in lipid and islet fibrotic areas in the OHR group were observed, along with increased insulin sensitivity and normalization of insulin-degrading enzyme levels. The expression of nicotinamide phosphoribosyltransferase (NAMPT), insulin receptor (IR), sirtuin 1 (SIRT1), and complex II proteins were increased in the liver tissue of the OHR group. Strong correlations, whether positive or negative, were evaluated via Spearman’s model between SIRT1, AMPK, NAMPT, PGC-1α, and NNMT expressions with the restoration of normal blood pressure, weight loss, glycemic and lipid panel, and mitochondrial adaptation.
Conclusion
CR provided short-term beneficial effects to recover the physiological parameters induced by a high-fat diet and renal artery stenosis in obese and hypertensive animals. These benefits, even in the short term, can provide physiological benefits in the long term.




Similar content being viewed by others
References
World Health Organization (2020) Obesity and overweight World Health Organization. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 1 Apr
Sung KC, Ryu S, Cheong ES et al (2015) All-cause and cardiovascular mortality among Koreans: effects of obesity and metabolic health. Am J Prev Med. https://doi.org/10.1016/j.amepre.2015.02.010
Saliba LJ, Maffett S (2019) Hypertensive heart disease and obesity: a review. Heart Fail Clin 15(4):509–517. https://doi.org/10.1016/j.hfc.2019.06.003
Felisbino-Mendes MS, Cousin E, Malta DC et al (2020) The burden of non-communicable diseases attributable to high BMI in Brazil, 1990–2017: findings from the global burden of disease study. Popul Health Metrics. https://doi.org/10.1186/s12963-020-00219-y
Hill JO, Wyatt HR, Peters JC (2012) Energy balance and obesity. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.111.087213
DiMenna FJ, Arad AD (2018) Exercise as ‘precision medicine’ for insulin resistance and its progression to type 2 diabetes: a research review. BMC Sports Sci Med Rehabil. https://doi.org/10.1186/s13102-018-0110-8
Röder PV, Wu B, Liu Y, Han W (2016) Pancreatic regulation of glucose homeostasis. Exp Mol Med 48(3):e219. https://doi.org/10.1038/emm.2016.6
Fontana L (2009) The scientific basis of caloric restriction leading to longer life. Curr Opin Gastroenterol. https://doi.org/10.1097/MOG.0b013e32831ef1ba
Speakman JR, Mitchell SE (2011) Caloric restriction. Mol Aspects Med 32(3):159–221. https://doi.org/10.1016/j.mam.2011.07.001
Kostogrys RB, Franczyk-Żarów M, Manterys A, Wybrańska I (2018) Effect of caloric restriction on liver function in young and old ApoE/LDLr-/- mice. Rocz Panstw Zakl Hig 69(1):37–43
Chung KW, Chung HY (2019) The effects of calorie restriction on autophagy: role on aging intervention. Nutrients 11(12):2923. https://doi.org/10.3390/nu11122923
Kume S, Uzu T, Horiike K et al (2010) Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Investig: https://doi.org/10.1172/JCI41376
Kraus D, Yang Q, Kong D et al (2014) Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. https://doi.org/10.1038/nature13198
Pillai VB, Sundaresan NR, Kim G et al (2013) Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circul Physiol. https://doi.org/10.1152/ajpheart.00468.2012
Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, Li C, Shen G, Zou B (2020) NAD+ metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther 5(1):227. https://doi.org/10.1038/s41392-020-00311-7
Mayurasakorn K, Hasanah N, Homma T et al (2018) Caloric restriction improves glucose homeostasis, yet increases cardiometabolic risk in caveolin-1-deficient mice. Metabo: Clin Exp. https://doi.org/10.1016/j.metabol.2018.01.012
Goldblatt H, Lynch J, Hanzal RF, Summerville WW (1934) Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal iscttemia. J Exp Med. https://doi.org/10.1084/jem.59.3.347
Buyukdere Y, Gulec A, Akyol A (2019) Cafeteria diet increased adiposity in comparison to high fat diet in young male rats. PeerJ. https://doi.org/10.7717/peerj.6656
Lo S, Russell JC, Taylor AW (1970) Determination of glycogen in small tissue samples. J Appl Physiol. https://doi.org/10.1152/jappl.1970.28.2.234
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Abreu-Filho PG, Tarragô AM, Costa AG et al (2019) Plasma eicosanoid profile in plasmodium vivax malaria: clinical analysis and impacts of self-medication. Front Immunol. https://doi.org/10.3389/fimmu.2019.02141
Ketonen J, Pilvi T, Mervaala E (2010) Caloric restriction reverses high-fat diet-induced endothelial dysfunction and vascular superoxide production in C57Bl/6 mice. Heart Vessels. https://doi.org/10.1007/s00380-009-1182-x
Gao X, Yan D, Zhao Y et al (2015) Moderate calorie restriction to achieve normal weight reverses β-cell dysfunction in diet-induced obese mice: Involvement of autophagy. Nutr Metab. https://doi.org/10.1186/s12986-015-0028-z
Hong S, Moreno-Navarrete JM, Wei X et al (2015) Nicotinamide N-methyltransferase regulates hepatic nutrient metabolism through Sirt1 protein stabilization. Nat Med. https://doi.org/10.1038/nm.3882
Kim KE, Jung Y, Min S et al (2016) Caloric restriction of db/db mice reverts hepatic steatosis and body weight with divergent hepatic metabolism. Sci Rep. https://doi.org/10.1038/srep30111
Müller MJ, Enderle J, Bosy-Westphal A (2016) Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. https://doi.org/10.1007/s13679-016-0237-4
Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11(2):98–107. https://doi.org/10.1038/nri2925
Delghingaro-Augusto V, Madad L, Chandra A et al (2014) Islet inflammation, hemosiderosis, and fibrosis in intrauterine growth-restricted and high fat-fed sprague-dawley rats. Am J Pathol. https://doi.org/10.1016/j.ajpath.2014.01.024
Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J (2017) Mitochondrial complex II: at the crossroads. Trends Biochem Sci 42(4):312–325. https://doi.org/10.1016/j.tibs.2017.01.003
Guidotti A, Baraldi M, Costa E (1979) 1,4-benzodiazepines and gamma-aminobutyric acid: Pharmacological and biochemical correlates. Pharmacology. https://doi.org/10.1159/000137322
Wei X, Ke B, Zhao Z et al (2014) Regulation of insulin degrading enzyme activity by obesity-associated factors and pioglitazone in liver of diet-induced obese mice. PLoS ONE. https://doi.org/10.1371/journal.pone.0095399
Nicol LE, Grant WF, Comstock SM et al (2013) Pancreatic inflammation and increased islet macrophages in insulin-resistant juvenile primates. J Endocrinol. https://doi.org/10.1530/JOE-12-0424
Castell-Auvá- A, Cedó LD, Pallarès V et al (2012) The effects of a cafeteria diet on insulin production and clearance in rats. Br J Nutr. https://doi.org/10.1017/S0007114511006623
Pillai VB, Sundaresan NR, Kim G et al (2010) Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. https://doi.org/10.1074/jbc.M109.077271
Venturini PR, Thomazini BF, Oliveira CA et al (2018) Vitamin e supplementation and caloric restriction promotes regulation of insulin secretion and glycemic homeostasis by different mechanisms in rats. Biochem Cell Biol. https://doi.org/10.1139/bcb-2018-0066
Choi SE, Fu T, Seok S et al (2013) Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. https://doi.org/10.1111/acel.12135
Picard F, Kurtev M, Chung N et al (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. https://doi.org/10.1038/nature02583
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol 298(4):E751–E760. https://doi.org/10.1152/ajpendo.00745.2009
Cao Y, Jiang X, Ma H, Wang Y, Xue P, Liu Y (2016) SIRT1 and insulin resistance. J Diabetes Complic 30(1):178–183. https://doi.org/10.1016/j.jdiacomp.2015.08.022
Revollo JR, Körner A, Mills KF et al (2007) Nampt/PBEF/Visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme. Cell Metab. https://doi.org/10.1016/j.cmet.2007.09.003
Yoshino J, Mills KF, Yoon MJ, Imai SI (2011) Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. https://doi.org/10.1016/j.cmet.2011.08.014
Yang H, Yang T, Baur JA et al (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. https://doi.org/10.1016/j.cell.2007.07.035
Tao R, Wei D, Gao H et al (2011) Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene. J Biol Chem. https://doi.org/10.1074/jbc.M110.201061
McCarty MF (2005) AMPK activation as a strategy for reversing the endothelial lipotoxicity underlying the increased vascular risk associated with insulin resistance syndrome. Med Hypotheses. https://doi.org/10.1016/j.mehy.2004.01.042
Acknowledgements
The authors are grateful to Edvaldo Costa, BSc, Sr Mateus Eduardo Bortolanza da Silva, Sra Ana Cristina Pires Menegheti, and Renata Barbieri for their excellent technical assistance.
Funding
This work was supported by a grant from the Pesquisa Instuticional Uniararas (PROPesq-FHO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this research was performed without any conflicts of interest or commercial or financial gains.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Preprint
Manuscript submitted to a pre-print platform Research Square, the https://doi.org/10.21203/rs.3.rs1085770/v1 and this work is licensed under a CC BY 4.0 License and pre-print plataform bioRxiv 2021.03.30.437684; https://doi.org/10.1101/2021.03.30.437684.
Supplementary Information
Below is the link to the electronic supplementary material.
11033_2022_7370_MOESM1_ESM.docx
Supplementary file1 (DOCX 282 kb). Figure 1: (A) Hepatic Glycogen and (B) Muscle Glycogen. Data expressed for SHAM, OH, and OHR groups. The bars represent the mean ± SEM, p<0.05 indicates a significant difference based on one-way ANOVA, Tukey post hoc test. Figure 2: Protein expression in the liver tissues of animals of the SHAM, OH, and OHR groups: (A) pAkt/Akt, (B) PGC1α, (C) FOXO, (D) SIRT3 and (E) NNMT. The bars represent the mean ± SEM, p<0.05 indicates a significant difference based on one-way ANOVA, Tukey post hoc test.
Rights and permissions
About this article
Cite this article
de Souza Nunes Faria, M.S., Pimentel, V.E., Helaehil, J.V. et al. Caloric restriction overcomes pre-diabetes and hypertension induced by a high fat diet and renal artery stenosis. Mol Biol Rep 49, 5883–5895 (2022). https://doi.org/10.1007/s11033-022-07370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07370-9