Abstract
Background
Compared with typical visceral fat deposits in obesity and metabolic syndrome, perirenal adipose tissue (PRAT) dysfunction is more closely linked to obesity-related chronic kidney disease (OB-CKD). The myokine irisin reportedly promotes positive outcomes in metabolic disease. This study investigated whether irisin could reduce urinary albumin excretion and demonstrate renoprotective effects through the regulation of PRAT function in obese mice.
Methods
C57BL/6 J mice received a high-fat diet (HFD) with or without concurrent administration of irisin. Glucose tolerance, plasma levels of free fatty acids, and urinary albumin excretion were assessed, along with renal morphology. The vascular endothelial growth factor and nitric oxide in glomeruli were also analyzed, in addition to PRAT function-associated proteins.
Results
Irisin administration significantly reduced the final body weight, fat mass, and free fatty acids, without reducing PRAT mass, in HFD mice. Furthermore, irisin decreased urinary albumin excretion and attenuated both renal fibrosis and lipid accumulation. Irisin administration led to increases in PRAT function-associated proteins, including sirtuin1, uncoupling protein-1, and heme-oxygenase-1. Ex vivo treatment of PRAT and glomeruli with irisin also restored PRAT function. Finally, irisin treatment restored the vascular endothelial growth factor–nitric oxide axis.
Conclusions
Irisin attenuated metabolic disorders and protected against OB-CKD by normalizing the PRAT–kidney axis. These results suggest that agents targeting PRAT activation might be useful for treatment of OB-CKD.
Similar content being viewed by others
Background
Obesity-related chronic kidney disease (OB-CKD) is receiving increasing attention because of the obesity pandemic [1,2,3]. OB-CKD is characterized by glomerular hypertrophy and microalbuminuria, which are also markers of systemic vascular endothelial cell lesions that involve renal arteries and glomerular endothelial cells [4, 5]. Thus, microalbuminuria links CKD to ischemic cardiovascular events [6]. Recently, research into obesity-related complications has shifted from adipose tissue distribution to the localized presence of adipose tissue around organs, including epicardial adipose tissue, perivascular adipose tissue (PVAT), and perirenal adipose tissue (PRAT) [7]. PRAT, which directly surrounds the kidneys and is closely associated with renal tissue, was originally thought to provide mechanical support alone. However, PRAT has been associated with OB-CKD [8, 9]. A previous study revealed that the presence of PRAT was predictive of microalbuminuria in obese patients [10]. PRAT can release various adipokines (e.g., adiponectin, interleukin-6, and leptin) that participate in early OB-CKD through effects on endothelial function in the renal vasculature and glomeruli [11]. The mechanism may be related to renal lipotoxicity and inflammatory infiltration. Moreover, unlike conventional visceral fat, PRAT comprises a combination of white and brown adipose tissues (WAT and BAT) [12]. PRAT can also be converted to BAT under some conditions [13]. Thus, PRAT offers a potential therapeutic target for OB-CKD.
Irisin, discovered in 2012, is a hormone secreted both by myocytes and adipose tissue [14]. Irisin can activate peroxisome proliferator-activated receptor (PPAR)α and broadly promote gene expression (e.g., uncoupling protein-1 [UCP-1]) in adipocytes. Irisin can also promote the phenotypic transformation of white adipocytes to brown adipocytes, thereby increasing free fatty acid (FFA) oxidation and energy consumption [15]. Because of these characteristics, irisin is presumed to have a connection with metabolic disease [16]. Elevated irisin secretion leads to increased energy expenditure, suggesting that irisin can modulate glucose homeostasis and be used for the treatment of obesity [17]. Additionally, irisin has been associated with improvements in cardiometabolic disturbances and cardiovascular disease [18]. Irisin overexpression protects vascular function by regulating endothelial and PVAT functions in obese mice [19, 20]. Patients with CKD usually have lower irisin levels, suggesting that a low level of irisin is a risk factor for CKD [21, 22]. Irisin also alleviates renal injury by improving urinary albumin [23]. However, it has been unclear whether irisin protects against OB-CKD by regulating PRAT function. The renoprotective effects of irisin in OB-CKD may be mediated by changes in PRAT function. This study investigated the impacts of irisin on urinary albumin excretion (UAE) in mice with high-fat diet (HFD)-induced obesity, then explored the mechanisms underlying its protective effects.
Methods
Experimental animals
Six-week-old male C57BL/6 J mice (Pengyue, Jinan, China) were assigned to three groups (n = 8/group). Control mice were fed a standard rodent chow, whereas HFD mice were fed an HFD (60% fat, 460 kcal/100 g) for 28 weeks. Irisin mice were fed an HFD for 28 weeks, and recombinant irisin (250 μg/kg; Phoenix Pharmaceuticals, USA) was intraperitoneally injected during the final 4 weeks. The mice were housed in standard environmentally controlled rooms and subjected to weekly measurements of body weight. Fat mass was measured using a Body Composition Analyzer (Bruker, Germany). The study protocol was approved by the Animal Ethics Committee at Weifang Medical University.
Biochemical testing
After 28 weeks of treatment as described above, the mice were subjected to glucose tolerance and insulin sensitivity tests, as previously described [24]. Blood samples and plasma were collected. Plasma glucose and FFAs were determined by the glucose oxidase and colorimetric assays, respectively. Twenty-four-hour urine collection was performed using metabolic cages; urinary albumin and creatinine were then measured using the assay kits (Exocell Inc., USA; Jiancheng, China), respectively.
Histology and immunohistochemistry
To evaluate morphological changes, left renal tissues were fixed in 10% formalin and embedded in paraffin. Coronal sections were collected from left kidney for histopathological examination via hematoxylin and eosin staining (Solarbio, China). To assess fibrosis, renal tissue sections were stained with picrosirius red (Sigma-Aldrich) [24]. Lipid accumulation in renal tissue sections was evaluated by Oil Red O staining [25]. Images were observed using light microscopy (magnification 400 × , Nikon, Tokyo, Japan) and analyzed by ImageJ software. Images of the cortex and medulla (3–4 images per region) from four mice in each group were analyzed.
Preparation of PRAT-derived conditioned medium (PRAT-CM)
For ex vivo studies, PRAT was collected in the form of PRAT-CM, as previously described [26]. Briefly, the PRAT was separated from renal tissue, washed, and incubated at 37 °C. The PRAT-CM was then stored at -80 °C. Glomeruli were isolated, then incubated with irisin for 48 h in PRAT-CM before measurement of protein levels [27]. Vascular endothelial growth factor (VEGF) secretion from glomeruli was determined by assay kits from R&D Systems (USA).
Western blotting analysis
Equivalent amounts of proteins from PRAT or glomeruli were homogenized, separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and then incubated with the following antibodies: anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), anti-heme oxygenase (HO-1), anti-sirtuin1 (SIRT1), and anti-UCP-1 (all from Cell Signaling Technology, USA), as well as anti-VEGF (Santa Cruz Biotechnology, USA). Subsequently, the blots were incubated with secondary antibody (Cell Signaling Technology, USA) and detected with Bio-Rad Laboratories (Hercules, CA, USA). Protein levels based on band intensity were quantified by ImageJ software. Band intensities were normalized to the intensity of the GAPDH band.
Quantitative polymerase chain reaction (qPCR) analysis
Total RNA from the tissues was isolated using a Pure Link RNA mini kit (Invitrogen, USA) and reverse-transcribed to cDNA using the PrimeScript RT reagent Kit with gDNA Eraser. Primers for quantitative qPCR were as follows: tumor necrosis factor (Tnf)-α, forward (CCTGTAGCCCACGTCGTAG) and reverse (GGGAGTAGACAAGGTACAACCC); monocyte chemoattractant protein-1 (Mcp-1), forward (TAAAAACCTGGATCGGAACCAAA) and reverse (GCATTAGCTTCAGATTTACGGGT).
Measurements of glomerular nitric oxide (NO) and mitochondrial reactive oxygen species (ROS)
Glomeruli were isolated and collected by a gradual sieving technique [27]. Total glomerular NO levels were determined by the Griess method [28]. Mitochondrial ROS production was assessed by MitoSOX Red (Invitrogen) [24].
Statistical analysis
Data are shown as means ± standard errors of the mean; they were analyzed using GraphPad 8.0. Statistical analysis was performed by one-way or two-way analysis of variance, and interaction effects were determined by the Tukey test. The threshold of P < 0.05 was considered statistically significant.
Results
Irisin alleviated metabolic disorders in HFD mice
As expected, exposure to an HFD led to significant increases in body weight (1.52-fold), fat mass (5.51-fold), and PRAT mass (10.73-fold; all P < 0.05). Irisin treatment prevented further body weight and fat gain (P < 0.05), but not PRAT gain (P > 0.05; Fig. 1A–E). Glucose tolerance and insulin sensitivity were substantially lower in HFD mice. Irisin administration to HFD mice improved glucose homeostasis and reduced FFA levels (P < 0.05; Fig. 1F–H). Thus, irisin improved metabolic parameters in HFD mice.
Irisin alleviated renal injury in HFD mice
Changes in renal structure and function were examined to evaluate the protective effects of irisin on OB-CKD. Twenty-four-hour UAE was substantially greater in HFD mice than in control mice (48.52 ± 22.83 µg/24 h vs. 11.88 ± 2.94 µg/24 h, P < 0.05; Fig. 2A). Compared with HFD mice, irisin mice exhibited 70.9% lower UAE (45.16 ± 10.21 µg/24 h vs. 14.11 ± 4.30 µg/24 h, P < 0.05; Fig. 2A). Similar to the UAE findings, irisin significantly reduced the albumin to creatinine ratio in HFD mice (5.31 ± 2.93 µg/µmol vs. 20.86 ± 10.24 µg/µmol; Fig. 2B). No significant differences were seen in creatinine (P > 0.05; Fig. 2C). Furthermore, HFD mice exhibited glomerular hypertrophy (glomerular area increased by 1.25-fold; P < 0.05; Fig. 2D, E), noticeable mesangial proliferation, glomerular fibrosis (% area increased by 92.86%; Fig. 2D, F), and significant lipid accumulation (% area increased by nine-fold; Fig. 2D, G). The abnormal pathological alterations were partially reversed by irisin administration (Fig. 2D–G). Thus, irisin alleviated HFD-induced renal injury.
Irisin alleviated renal injury in HFD mice. A 24-h urinary albumin excretion; B albumin to creatinine ratio; C serum creatinine; D H&E, picrosirius red and oil red o staining; E–G quantitative analysis for glomerular area, area occupied by lipid droplets and fibrosis area. *P < 0.05; n = 6/group for panel A-C; n = 3–4 slices from four mice/group panel E–G
Irisin mediated kidney protection by regulating the glomerular VEGF–NO axis
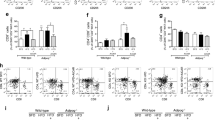
A previous study demonstrated that increased uncoupling of VEGF–NO axis was the main mechanism underlying the onset of OB-CKD [29]. As expected, glomerular VEGF levels were increased and NO production was reduced in HFD mice. Notably, irisin reduced VEGF levels by 74.4% and enhanced NO production by 63.8%, thus restoring the glomerular VEGF–NO axis (P < 0.05; Fig. 3A, B). To clarify whether irisin directly protected the PRAT-modified glomerular VEGF–NO axis, ex vivo studies involving PRAT-CM were performed. Treatment of glomeruli (for 48 h) with PRAT-CM collected from HFD mice led to higher VEGF levels/secretion (1762 ± 41 pg/ml vs.1175 ± 43 pg/ml) and lower NO production (P < 0.05). However, treatment with irisin partially restored normal VEGF–NO axis activity (VEGF reduction by 46.0%, VEGF secretion reduction by 38.27% with 1274 ± 41 pg/ml vs. 1762 ± 41 pg/ml, and NO increase by 63.8%; P < 0.05; Fig. 3C, D). Notably, this restoration of VEGF–NO axis activity was reversed by treatment with irisin and the PPARα inhibitor GW6471 (10 μM), which blocked irisin-mediated browning of adipocytes (Fig. 3C, D). Taken together, these findings indicate that irisin mediated kidney protection by regulating the glomerular VEGF–NO axis.
Irisin activated UCP-1, SIRT1, and HO-1 in PRAT from HFD mice
PRAT was collected from mice to explore whether these beneficial effects on OB-CKD were associated with PRAT function. The activation of UCP-1 and the activation of SIRT1 are both associated with the browning of WAT [30]; the activation of HO-1 is associated with adipocyte reprogramming to demonstrate characteristics of browning [31]. As expected, HFD mice exhibited reduced the levels of UCP-1, SIRT1, and HO-1 in PRAT. These proteins in PRAT were enhanced after irisin administration (P < 0.05; Fig. 4C, D). To further investigate whether irisin directly affects these proteins, HFD mouse-derived PRAT was treated with irisin (48 h) ex vivo; the corresponding pathways were then evaluated in PRAT homogenates by western blotting. As expected, irisin treatment significantly activated all three pathways in PRAT (Fig. 4C, D). Similar to the findings in the previous section, these alterations were partially reversed by treatment with the PPARα inhibitor. Thus, irisin activated UCP-1, SIRT1, and HO-1 in PRAT from HFD mice.
Irisin attenuated the production of ROS and inflammatory cytokines in PRAT
OB-CKD is associated with oxidative injury and inflammatory infiltration. To determine whether irisin could alleviate the production of ROS and inflammatory cytokines, mitochondrial ROS production was evaluated using the MitoSOX Red fluorescent probe. HFD mice exhibited robust production of mitochondrial ROS, Tnf-α, and Mcp-1, along with reduced mitochondrial DNA content, in renal tissue. Irisin treatment resulted in 50.57% reduction in mitochondrial ROS (P < 0.05; Fig. 5A, B), 62.98% reduction in Tnf-α, and 48.61% reduction in Mcp-1, as well as 64.59% enhancement of mitochondrial DNA content (P < 0.05; Fig. 5C).
Discussion
The mechanisms underlying the development of OB-CKD involve lipotoxicity, enhanced oxidative stress, and the activation of various inflammatory factors. OB-CKD onset is mainly associated with the accumulation of adipose tissues, such as PRAT. This study demonstrated that irisin could reduce UAE, albumin to creatinine ratio and attenuate renal injury in HFD mice. These findings were related to the browning of PRAT and restoration of glomerular VEGF–NO axis activity. These findings indicate that the renoprotective effects of irisin in obesity are mediated by the regulation of PRAT function.
Obesity-related complications have received considerable attention in the past decade. The onset of obesity leads to metabolic alterations that promote cardiovascular and renal diseases. In this study, receipt of an HFD for 28 weeks led to obvious metabolic abnormalities, including increased body weight, fat, and FFA levels. A previous study demonstrated that PRAT is related to OB-CKD [11]. PRAT surrounds the kidneys and renal vessels; it thus has regulatory effects on renal function. PRAT accumulation can lead to increased FFA release; this induced lipotoxicity impairs renal function and exaggerates renal injury [10, 11]. In the present study, HFD promoted PRAT accumulation and enhanced the levels of circulating FFAs and renal lipids, thus amplifying renal injury in the form of increased UAE, glomerular hypertrophy, renal fibrosis, and lipid droplet accumulation.
Irisin is generated by the proteolytic processing of fibronectin type III domain-containing protein 5; this newly discovered hormone is mainly secreted by myocytes. Irisin affects energy and glucose metabolism; thus, it is potentially useful in treatments for obesity and associated metabolic alterations. Irisin may protect against cardiovascular disease [32, 33]. Hu et al. found that irisin administration significantly suppressed activation of the cardiac inflammasome, thus attenuating age-related cardiac dysfunction [32]. Yu et al. found that irisin attenuates pressure overload-induced cardiac hypertrophy, mainly by regulating adenosine monophosphate-activated protein kinase. With regard to kidney disease, circulating irisin levels have been reported to decrease with increasing CKD severity [34]. Furthermore, irisin alleviates sepsis-induced renal injury by inhibiting the NF-κB [35]. Currently, there are no published reports concerning the effects of irisin on OB-CKD. This study showed that irisin can improve metabolic abnormalities and reduce both UAE and renal injury in obese mice, indicating that irisin can protect against OB-CKD.
The effects of irisin on OB-CKD may be explained as follows. Unlike other types of CKD, OB-CKD is usually the initial step of renal injury; it principally involves vascular disorders [36, 37]. A previous study demonstrated that this early renal injury is associated with the PRAT-derived FFA-induced impaired glomerular VEGF–NO axis in obesity [11, 29]. Reduced NO production, thereby increasing VEGF production. The overproduction of VEGF could increase UAE by promoting vessel proliferation and permeability. This study showed that irisin treatment significantly increased NO production and decreased glomerular VEGF levels. Importantly, the treatment of glomeruli with HFD-derived PRAT-CM led to decreased NO production and increased VEGF levels. However, treatment with irisin restored normal VEGF–NO axis activity. These findings indicated that the PRAT-modified abnormal VEGF–NO axis participated in OB-CKD; these abnormalities could be reversed by irisin in a manner that did not require metabolic improvement.
In contrast to other visceral fat, PRAT has a “bright” characteristic and contains both BAT and WAT; it has the potential to transition from BAT into WAT. This potential was confirmed by Efremova et al., who reported that approximately 30% of PRAT expressed UCP-1 [38]. A previous study demonstrated that irisin could promote brown-like fat in PVAT, another adipose tissue with a “bright” characteristic [20]. The present study analyzed PRAT browning to determine whether it is associated with the ability of irisin to protect against OB-CKD. Notably, treatment with irisin led to increased UCP-1 and SIRT1 in PRAT, indicating that irisin had caused browning of PRAT. Notably, this protective effect was reversed by inhibiting the browning of PRAT, as demonstrated by the ability of the PPARα inhibitor co-administered with irisin to hinder restoration of the glomerular VEGF–NO axis.
Adipose browning is associated with thermogenesis and the regulation of energy balance. This white-to-brown transdifferentiation promotes several metabolism-modifying factors; these include the anti-oxidative protein HO-1, which protects against cardiovascular disease through powerful anti-oxidative and anti-inflammatory properties [39]. Additionally, irisin-mediated vessel protection is associated with enhanced HO-1 production by PVAT. In PRAT around blood vessels and surrounding the kidneys, HO-1 production can be regulated by irisin. Activation of this pathway attenuates ROS production and inflammation; decreases in ROS production and inflammation could prevent the use of NO to form peroxynitrite, thus enhancing NO bioavailability. In the present study, treatment with irisin led to increased HO-1 production, both in vivo and ex vivo. Furthermore, after irisin treatment, the levels of mitochondrial ROS and inflammatory factors were substantially reduced. Previous studies have also shown that irisin enhances other anti-oxidative enzymes including superoxide dismutase, glutathione peroxidase, and catalase [40, 41]. These encouraging results indicate that PRAT-derived HO-1 regulates PRAT function; irisin could regulate PRAT function by enhancing the HO-1 system.
Comparisons with other studies and what does the current work add to the existing knowledge
PRAT dysfunction has previously demonstrated a close association with OB-CKD. The present study demonstrated that irisin-mediated regulation of PRAT function could protect against OB-CKD.
Study strengths and limitations
The strength of the study is that it highlights the potential for protecting against OB-CKD by using agents that target PRAT function. This study also had some limitations. Because irisin treatment significantly decreased body weight and fat, the irisin-related alleviation of OB-CKD may have been secondary to metabolic improvement. However, irisin also altered these protein levels in ex vivo experiments, indicating that irisin could protect against OB-CKD by regulating PRAT function in a manner that did not require metabolic alteration. Additionally, the delivery and distribution of irisin in PRAT and glomeruli were not investigated; the use of an irisin receptor blocker (e.g., CycloRGDyK) would provide greater support to the findings [42, 43]. Finally, PRAT function was solely monitored by changes in its mass and the levels of some essential proteins. Future PRAT activity/mass assessments should include fluorodeoxyglucose positron emission tomography/computed tomography.
Conclusions
In summary, this study showed that the receipt of an HFD exacerbates PRAT dysfunction and kidney injury, including decreases in UCP-1/SIRT1 levels and increases in both UAE and lipid accumulation. Irisin was able to protect against OB-CKD by regulating the PRAT–kidney axis; the effects included browning of PRAT and restoration of the VEGF–NO axis. These results indicate that agents targeting PRAT activation might be useful for treatment of OB-CKD.
Availability of data and materials
Data will be made available on request.
References
Pinto K, Feckinghaus CM, Hirakata VN. Obesity as a predictive factor for chronic kidney disease in adults: systematic review and meta-analysis. Braz J Med Biol Res. 2021;54:e10022.
Spatola L, Dozio E. Obesity-associated mortality risk: Chronic kidney disease in focus. Nutr Metab Cardiovasc Dis. 2019;29:212–3.
Joyce T, Chirino YI, Natalia MT, Jose PC. Renal damage in the metabolic syndrome (MetSx): Disorders implicated. Eur J Pharmacol. 2018;818:554–68.
Han F, Hou N, Miao W, Sun X. Correlation of ultrasonographic measurement of intrarenal arterial resistance index with microalbuminuria in nonhypertensive, nondiabetic obese patients. Int Urol Nephrol. 2013;45:1039–45.
Tesauro M, Canale MP, Rodia G, Di Daniele N, Lauro D, Scuteri A, Cardillo C. Metabolic syndrome, chronic kidney, and cardiovascular diseases: role of adipokines. Cardiol Res Pract. 2011;2011:653182.
Souweine JS, Corbel A, Rigothier C, Roque CD, Hadjadj S, Cristol JP, Combe C, Bigot-Corbel E, Beauvieux MC. Interest of albuminuria in nephrology, diabetology and as a marker of cardiovascular risk. Ann Biol Clin (Paris). 2019;77:26–35.
Martin-Taboada M, Vila-Bedmar R, Medina-Gómez G. From Obesity to Chronic Kidney Disease: How Can Adipose Tissue Affect Renal Function. Nephron. 2021;145:609–13.
Huang N, Mao EW, Hou NN, Liu YP, Han F, Sun XD. Novel insight into perirenal adipose tissue: A neglected adipose depot linking cardiovascular and chronic kidney disease. World J Diabetes. 2020;11:115–25.
Grigoraș A, Balan RA, Căruntu ID, Giușcă SE, Lozneanu L, Avadanei RE, Rusu A, Riscanu LA, Amalinei C. Perirenal Adipose Tissue-Current Knowledge and Future Opportunities. J Clin Med. 2021;10:1291.
Sun X, Han F, Miao W, Hou N, Cao Z, Zhang G. Sonographic evaluation of para- and perirenal fat thickness is an independent predictor of early kidney damage in obese patients. Int Urol Nephrol. 2013;45:1589–95.
Hou N, Han F, Wang M, Huang N, Zhao J, Liu X, Sun X. Perirenal fat associated with microalbuminuria in obese rats. Int Urol Nephrol. 2014;46:839–45.
Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol. 2012;112:1008–14.
Jespersen NZ, Feizi A, Andersen ES, Heywood S, Hattel HB, Daugaard S, Peijs L, Bagi P, Feldt-Rasmussen B, Schultz HS, et al. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol Metab. 2019;24:30–43.
Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Chen N, Li Q, Liu J, Jia S. Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev. 2016;32:51–9.
Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–37.
Arhire LI, Mihalache L, Covasa M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front Endocrinol (Lausanne). 2019;10:524.
Han F, Zhang S, Hou N, Wang D, Sun X. Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol. 2015;309:H1501-1508.
Hou N, Liu Y, Han F, Wang D, Hou X, Hou S, Sun X. Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese mice. J Mol Cell Cardiol. 2016;99:188–96.
Liu JJ, Liu S, Wong MD, Tan CS, Tavintharan S, Sum CF, Lim SC. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J Diabetes Complicat. 2014;28:208–13.
Sadeghi Shad J, Akbari R, Qujeq D, Hajian-Tilaki K. Measurement of serum irisin in the different stages of chronic kidney disease. Caspian J Intern Med. 2019;10:314–9.
Peng H, Wang Q, Lou T, Qin J, Jung S, Shetty V, Li F, Wang Y, Feng XH, Mitch WE, et al. Myokine mediated muscle-kidney crosstalk suppresses metabolic reprogramming and fibrosis in damaged kidneys. Nat Commun. 2017;8:1493.
Sun X, Han F, Lu Q, Li X, Ren D, Zhang J, Han Y, Xiang YK, Li J. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69:1292–305.
Escorcia W, Ruter DL, Nhan J, Curran SP. Quantification of lipid abundance and evaluation of lipid distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J Vis Exp. 2018:57352.
Hou N, Du G, Han F, Zhang J, Jiao X, Sun X. Irisin regulates Heme Oxygenase-1/Adiponectin Axis in perivascular adipose tissue and improves endothelial dysfunction in diet-induced obese mice. Cell Physiol Biochem. 2017;42:603–14.
Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia. 2011;54:979–88.
Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–8.
Sun X, Yu Y, Han L. High FFA levels related to microalbuminuria and uncoupling of VEGF-NO axis in obese rats. Int Urol Nephrol. 2013;45:1197–207.
Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620–32.
Singh SP, Grant I, Meissner A, Kappas A, Abraham NG. Ablation of adipose-HO-1 expression increases white fat over beige fat through inhibition of mitochondrial fusion and of PGC1α in female mice. Horm Mol Biol Clin Investig. 2017;31.
Hu C, Zhang X, Hu M, Teng T, Yuan YP, Song P, Kong CY, Xu SC, Ma ZG, Tang QZ. Fibronectin type III domain-containing 5 improves aging-related cardiac dysfunction in mice. Aging Cell. 2022, 21:e13556.
Yu Q, Kou W, Xu X, Zhou S, Luan P, Xu X, Li H, Zhuang J, Wang J, Zhao Y, et al. FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin Sci (Lond). 2019;133:611–27.
Ebert T, Focke D, Petroff D, Wurst U, Richter J, Bachmann A, Lössner U, Kralisch S, Kratzsch J, Beige J, et al. Serum levels of the myokine irisin in relation to metabolic and renal function. Eur J Endocrinol. 2014;170:501–6.
Jin YH, Li ZY, Jiang XQ, Wu F, Li ZT, Chen H, Xi D, Zhang YY, Chen ZQ. Irisin alleviates renal injury caused by sepsis via the NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:6470–6.
Sorop O, Olver TD, van de Wouw J, Heinonen I, van Duin RW, Duncker DJ, Merkus D. The microcirculation: a key player in obesity-associated cardiovascular disease. Cardiovasc Res. 2017;113:1035–45.
Whaley-Connell A, Sowers JR. Obesity and kidney disease: from population to basic science and the search for new therapeutic targets. Kidney Int. 2017;92:313–23.
Efremova A, Senzacqua M, Venema W, Isakov E, Di Vincenzo A, Zingaretti MC, Protasoni M, Thomski M, Giordano A, Cinti S. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem. 2020;76:185–92.
Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–9.
Mazur-Bialy AI, Kozlowska K, Pochec E, Bilski J, Brzozowski T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J Physiol Pharmacol. 2018;69:117–25.
Mazur-Bialy AI, Pocheć E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants (Basel). 2021;10(1):88.
Formigari GP, Dátilo MN, Vareda B, Bonfante I, Cavaglieri CR, Lopes de Faria JM, Lopes de Faria JB. Renal protection induced by physical exercise may be mediated by the Irisin/AMPK axis in diabetic nephropathy. Sci Rep. 2022;12:9062.
Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, et al. Irisin mediates effects on bone and fat via αV integrin receptors. Cell. 2018;175:1756-1768.e17.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81870593 and 82170865), Natural Science Foundation of Shandong Province of China (ZR2020MH106) and Yuandu Scholar (2021).
Author information
Authors and Affiliations
Contributions
FH and CXK analyzed the data and drafted the manuscript. XDS and NNH designed the study and participated in the revision. The other authors participated in partial data collection and analysis. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Animal Ethics Committee at Weifang Medical University.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Han, F., Kan, C., Wu, D. et al. Irisin protects against obesity-related chronic kidney disease by regulating perirenal adipose tissue function in obese mice. Lipids Health Dis 21, 115 (2022). https://doi.org/10.1186/s12944-022-01727-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-022-01727-6