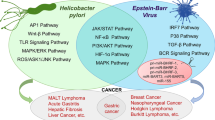
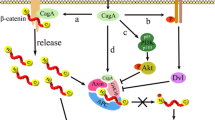
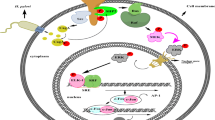
Abstract
Introduction
Many pathogens have coexisted with humans for millennia and can cause chronic inflammation which is the cause of gastritis. Gastric cancer (GC) is associated with 8.8% of cancer related deaths, making it one of the leading causes of cancer related deaths worldwide. This review is intended to give brief information about Helicobacter pylori (H. pylori), Epstein–Barr virus (EBV), human cytomegalovirus (HCMV) role in GC and associated kinases. These organisms can trigger multiple cellular pathways aiming for unnatural cellular proliferation, apoptosis, migration and inflammatory response. Kinases also can activate and deactivate the signalling leading to aforementioned pathways. Therefore, studying kinases is inevitable.
Material and methods
This review is the comprehensive collection of information from different data sources such as journals, book, book chapters and verified online information.
Conclusion
Kinase amplifications could be used as diagnostic, prognostic, and predictive biomarkers in various cancer types. Hence targeting kinase and related signalling molecules could be considered as a potential approach to prevent cancer through these organisms. Here we summarize the brief information about the role of kinases, signalling and their therapeutics in GC concerning H. pylori, EBV and HCMV.



Similar content being viewed by others
Abbreviations
- ARID1A:
-
AT-rich interactive domain-containing protein 1A
- Bcl-2:
-
B-Cell CLL/Lymphoma 2
- BabA:
-
Blood group antigen binding adhesin A
- cas-9:
-
Caspase-3 (cas-3), caspase-9
- CCDC151:
-
Coiled-coil domain containing 151
- CHRNB2:
-
Cholinergic receptor nicotinic beta 2 subunit
- CIMP:
-
Concordant methylation of multiple genes/loci
- CLTs:
-
Cytomegalovirus latency-associated transcripts
- CagA:
-
Cytotoxin associated gene A
- cagPAI:
-
Cytotoxin-associated genes pathogenicity island
- COX-2:
-
Cyclooxygenase-2
- CTLs:
-
Cytotoxic T cells
- EBV:
-
Epstein–Barr virus
- EBVaGC:
-
EBV-associated GC
- EBNA-1:
-
EBV nuclear antigen-1
- EPHA4:
-
EPH receptor A4
- EGF:
-
Epidermal growth factor
- ERBB2:
-
Erb-B2 receptor tyrosine kinase
- ERBB4:
-
Receptor tyrosine-protein kinase erbB-4 precursor
- ERK1/2:
-
Extracellular signal-regulated kinase ½
- FGFR2:
-
Fibroblast growth factor receptor 2
- leb:
-
Fucosylated lewisb
- GC:
-
Gastric cancer
- GMPR2:
-
Guanosine monophosphate reductase 2
- HSP-70:
-
Heat shock protein-70
- H. pylori :
-
Helicobacter pylori
- HDGFRP2:
-
Hepatoma-derived growth factor-related protein 2 isoform 1
- LMP:
-
Latent membrane proteins
- IAPs:
-
Inhibitors of apoptosis
- HCMV:
-
Human cytomegalovirus
- HER2:
-
Human Epidermal growth factor Receptor 2
- IHH:
-
Indian hedgehog homolog
- ITK:
-
Inducible T-cell KINASE
- IARC:
-
International Agency for Research on Cancer
- AP-1:
-
Jun/Activator protein 1
- mTOR:
-
Mammalian target of rapamycin
- MMPs:
-
Matrix metalloproteases
- Met:
-
Mesenchymal-epithelial transition factor
- MLH1:
-
MutL Homolog 1
- nRTK:
-
Non-receptor tyrosine kinases
- NFκB:
-
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- PAK1:
-
P21 (RAC1) Activated Kinase 1
- PAK2:
-
PAK2 Gene—P21 (RAC1) Activated Kinase 2
- PLCγ:
-
Phospholipase Cγ
- PTEN:
-
Phosphatase and Tensin Homolog
- PIK3CA:
-
Phosphatidylinositol 4,5-bisphosphate 3-Kinase Catalytic subunit Alpha isoform
- PI3K:
-
Phosphatidylinositol 3-Kinase/Akt
- PD-1:
-
Programmed cell death 1
- PGE2:
-
Prostaglandin E2
- PML:
-
Progressive multifocal leukoencephalopathy
- Src:
-
Proto-oncogene tyrosine-protein kinase
- RTK:
-
Receptor tyrosine kinases
- RPTP:
-
Receptor protein tyrosine phosphatase
- MEK:
-
Ras/Raf/Mitogen-activated protein kinase/ERK kinase
- ERK:
-
Extracellular-signal-regulated kinase
- SSTR1:
-
Somatostatin Receptor 1
- SHP2:
-
Src homology region 2
- STAD:
-
Stomach adenocarcinoma
- TYK2:
-
Tyrosine kinase 2
- VacA:
-
Vacuolating Cytotoxin A
- VSTM2L:
-
V-set and transmembrane domain containing 2 like
- VEGFR:
-
VEGF receptor
- HAUSP:
-
Ubiquitin-specific protease USP7
- ZEB1:
-
Zinc finger E-box-binding homeobox 1
- TP53:
-
Tumour protein P53
References
Cicenas J, Zalyte E, Bairoch A, Gaudet P (2018) Kinases and cancer. Cancers (Basel) 10:63
Chichirau BE, Diechler S, Posselt G, Wessler S (2019) Tyrosine kinases in Helicobacter pylori infections and gastric cancer. Toxins (Basel). 11:591
Kamran M, Long Z-J, Xu D, Lv S-S, Liu B, Wang C-L et al (2017) Aurora kinase A regulates survivin stability through targeting FBXL7 in gastric cancer drug resistance and prognosis. Oncogenesis 6:e298–e298
Thrift AP, El-Serag HB (2020) Burden of gastric cancer. Clin Gastroenterol Hepatol 18:534–542
Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP (2018) Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res 10:239–248
Singh S, Jha HC (2017) Status of Epstein-Barr virus coinfection with Helicobacter pylori in gastric cancer. J Oncol. https://doi.org/10.1155/2017/3456264
Correa P, Piazuelo MB (2011) Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev 7:59
Liu D, Ma X, Yang F, Xiao D, Jia Y, Wang Y (2020) Discovery and validation of methylated-differentially expressed genes in Helicobacter pylori-induced gastric cancer. Cancer Gene Ther 27:473–485
Díaz P, Valenzuela Valderrama M, Bravo J, Quest AFG (2018) Helicobacter pylori and gastric cancer: adaptive cellular mechanisms involved in disease progression. Front Microbiol 9:5
Kashyap D, Baral B, Verma TP, Sonkar C, Chatterji D, Jain AK et al (2020) Oral rinses in growth inhibition and treatment of Helicobacter pylori infection. BMC Microbiol 20:45
Eichelberg MR, Welch R, Guidry JT, Ali A, Ohashi M, Makielski KR et al (2019) Epstein-Barr virus infection promotes epithelial cell growth by attenuating differentiation-dependent exit from the cell cycle. MBio 10:e01332
Yu J, Liang Q, Wang J, Wang K, Gao J, Zhang J et al (2017) REC8 functions as a tumor suppressor and is epigenetically downregulated in gastric cancer, especially in EBV-positive subtype. Oncogene 36:182–193
Fattahi S, Nikbakhsh N, Taheri H, Ghadami E, Kosari-Monfared M, Amirbozorgi G et al (2018) Prevalence of multiple infections and the risk of gastric adenocarcinoma development at earlier age. Diagn Microbiol Infect Dis 92:62–68
Falush D (2003) Traces of human migrations in Helicobacter pylori populations. Science 299:1582–1585
Nomura A, Stemmermann GN, Chyou P-H, Kato I, Perez-Perez GI, Blaser MJ (1991) Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 325:1132–1136
Chmiela M, Karwowska Z, Gonciarz W, Allushi B, Stączek P (2017) Host pathogen interactions in Helicobacter pylori related gastric cancer. World J Gastroenterol 23:1521–1540
Yılmaz N, Koruk-Özer M (2019) The prevalence of Helicobacter pylori babA, homB, aspA, and sabA genes and its relationship with clinical outcomes in Turkey. Can J Gastroenterol Hepatol. https://doi.org/10.1155/2019/1271872
Ilver D, Arnqvist A, Ögren J, Frick I-M, Kersulyte D, Incecik ET et al (1998) Helicobacter pylori adhesin binding fucosylated Histo-Blood Group antigens revealed by retagging. Science 279:373–377
Kable ME, Hansen LM, Styer CM, Deck SL, Rakhimova O, Shevtsova A et al (2017) Host determinants of expression of the Helicobacter pylori BabA adhesin. Sci Rep 7:46499
Bernard MD, Arico B, Papini E, Rizzuto R, Grandi G, Rappuoli R et al (1997) Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol 26:665–674
Foegeding NJ, Caston RR, McClain MS, Ohi MD, Cover TL (2016) An overview of Helicobacter pylori VacA toxin biology. Toxins 8:173
Zhu P, Xue J, Zhang Z, Jia Y, Tong Y, Han D et al (2017) Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis 8:1–12
Cover TL, Blanke SR (2005) Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol 3:320–332
Šterbenc A, Jarc E, Poljak M, Homan M (2019) Helicobacter pylori virulence genes. World J Gastroenterol 25:4870–4884
Richardson CJ, Gao Q, Mitsopoulous C, Zvelebil M, Pearl LH, Pearl FMG (2009) MoKCa database—mutations of kinases in cancer. Nucleic Acids Res 37:D824–D831
Tomb J-F, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD et al (1997) Erratum: the complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 389:412–412
Alm RA, Trust TJ (1999) Analysis of the genetic diversity of Helicobacter pylori: the tale of two genomes. J Mol Med 77:834–846
Chen S-Y, Zhang R-G, Duan G-C (2016) Pathogenic mechanisms of the oncoprotein CagA in H. pylori-induced gastric cancer (Review). Oncol Rep 36:3087–3094
Liu X, Ji Q, Zhang C, Liu X, Liu Y, Liu N et al (2017) miR-30a acts as a tumor suppressor by double-targeting COX-2 and BCL9 in H. pylori gastric cancer models. Sci Rep 7:7113
Liu N, Zhou N, Chai N, Liu X, Jiang H, Wu Q et al (2016) Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 16:321
Hu Y, He C, Liu J-P, Li N-S, Peng C, Yang-Ou Y-B et al (2018) Analysis of key genes and signaling pathways involved in Helicobacter pylori-associated gastric cancer based on The Cancer Genome Atlas database and RNA sequencing data. Helicobacter 23:e12530
Sasaki S, Nishikawa J, Sakai K, Iizasa H, Yoshiyama H, Yanagihara M et al (2019) EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer 22:486–496
Jha HC, Pei Y, Robertson ES (2016) Epstein-Barr virus: diseases linked to infection and transformation. Front Microbiol 7:1602. https://doi.org/10.3389/fmicb.2016.01602
Young LS, Arrand JR, Murray PG (2007) EBV gene expression and regulation. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R et al (eds) Human herpesviruses: biology, therapy, and immunoprophylaxis. Cambridge University Press, Cambridge
Jha HC, Yang K, El-Naccache DW, Sun Z, Robertson ES (2015) EBNA3C regulates p53 through induction of Aurora kinase B. Oncotarget Impact J 6:5788–5803
Jha HC, Lu J, Saha A, Cai Q, Banerjee S, Prasad MAJ et al (2013) EBNA3C-mediated regulation of aurora kinase B contributes to Epstein–Barr virus-induced B-cell proliferation through modulation of the activities of the retinoblastoma protein and apoptotic caspases. J Virol 87:12121–12138
Zhang R, Strong MJ, Baddoo M, Lin Z, Wang Y-P, Flemington EK et al (2017) Interaction of Epstein–Barr virus genes with human gastric carcinoma transcriptome. Oncotarget Impact J 8:38399–38412
Fukayama M (2010) Epstein-Barr virus and gastric carcinoma. Pathol Int 60:337–350
Kang BW, Baek DW, Kang H, Baek JH, Kim JG (2019) Novel therapeutic approaches for Epstein–Barr virus associated gastric cancer. Anticancer Res 39:4003–4010
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B et al (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209
Matsusaka K, Funata S, Fukayama M, Kaneda A (2014) DNA methylation in gastric cancer, related to Helicobacter pylori and Epstein–Barr virus. World J Gastroenterol 20:3916–3926
Geddert H, Hausen AZ, Gabbert HE, Sarbia M (2010) EBV-infection in cardiac and non-cardiac gastric adenocarcinomas is associated with promoter methylation of p16, p14 and APC, but not hMLH1. Anal Cell Pathol 33:143–149
Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R et al (2018) Outlooks on Epstein–Barr virus associated gastric cancer. Cancer Treat Rev 66:15–22
Miliotis CN, Slack FJ (2020) Multi-layered control of PD-L1 expression in Epstein–Barr virus-associated gastric cancer. J Cancer Meta Treat 6:13
Sonkar C, Verma T, Chatterji D, Jain AK, Jha HC (2020) Status of kinases in Epstein–Barr virus and Helicobacter pylori coinfection in gastric cancer cells. BMC Cancer 20:925
Sivachandran N et al (2020) Epstein–Barr nuclear antigen 1 hijacks the host kinase CK2 to disrupt PML nuclear bodies. J Virol. https://doi.org/10.1128/JVI.01183-10
Fukuda M, Longnecker R (2004) Latent membrane protein 2A inhibits transforming growth factor-β1-induced apoptosis through the phosphatidylinositol 3-Kinase/Akt pathway. J Virol 78:1697–1705
Fukuda M, Longnecker R (2007) Epstein–Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt pathway. J Virol 81:9299–9306
Hino R, Uozaki H, Inoue Y, Shintani Y, Ushiku T, Sakatani T et al (2008) Survival advantage of EBV-associated gastric carcinoma: survivin up-regulation by viral latent membrane protein 2A. Cancer Res 68:1427–1435
Qi Y-F, Liu M, Zhang Y, Liu W, Xiao H, Luo B (2019) EBV down-regulates COX-2 expression via TRAF2 and ERK signal pathway in EBV-associated gastric cancer. Virus Res 272:197735
Dávila-Collado R, Jarquín-Durán O, Dong LT, Espinoza JL (2020) Epstein-Barr virus and Helicobacter pylori co-infection in non-malignant gastroduodenal disorders. Pathogens 9:104
Pandey S, Jha HC, Shukla SK, Shirley MK, Robertson ES (2018) Epigenetic regulation of tumor suppressors by Helicobacter pylori enhances EBV-induced proliferation of gastric epithelial cells. MBio 9:e00649
Cárdenas-Mondragón MG, Carreón-Talavera R, Camorlinga-Ponce M, Gomez-Delgado A, Torres J, Fuentes-Pananá EM (2013) Epstein Barr virus and Helicobacter pylori co-infection are positively associated with severe gastritis in pediatric patients. PLoS ONE 8:e62850
Mohr CF, Kalmer M, Gross C, Mann MC, Sterz KR, Kieser A et al (2014) The tumor marker Fascin is induced by the Epstein–Barr virus-encoded oncoprotein LMP1 via NF-κB in lymphocytes and contributes to their invasive migration. Cell Commun Signal 12:46
Liu X, Cohen JI (2016) Epstein–Barr virus (EBV) tegument protein bgLF2 promotes EBV reactivation through activation of the p38 mitogen-activated protein kinase. J Virol 90:1129–1138
Pena-Ponce MG, Jimenez MT, Hansen LM, Solnick JV, Miller LA (2017) The Helicobacter pylori type IV secretion system promotes IL-8 synthesis in a model of pediatric airway epithelium via p38 MAP kinase. PLoS ONE 12:e0183324
Byun E, Park B, Lim JW, Kim H (2016) Activation of NF-κB and AP-1 mediates hyperproliferation by inducing β-catenin and c-Myc in Helicobacter pylori-infected gastric epithelial cells. Yonsei Med J 57:647–651
Saju P, Murata-Kamiya N, Hayashi T, Senda Y, Nagase L, Noda S et al (2016) Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein–Barr virus. Nat Microbiol 1:1–8
Sinclair J (2008) Human cytomegalovirus: latency and reactivation in the myeloid lineage. J Clin Virol 41:180–185
Slobedman B, Cao JZ, Avdic S, Webster B, McAllery S, Cheung AK et al (2010) Human cytomegalovirus latent infection and associated viral gene expression. Fut Microbiol 5:883–900
Del Moral-Hernández O, Castañón-Sánchez CA, Reyes-Navarrete S, Martínez-Carrillo DN, Betancourt-Linares R, Jiménez-Wences H et al (2019) Multiple infections by EBV, HCMV and Helicobacter pylori are highly frequent in patients with chronic gastritis and gastric cancer from Southwest Mexico: an observational study. Medicine 98:e14124
Soroceanu L, Cobbs CS (2011) Is HCMV a tumor promoter? Virus Res 157:193–203
Zhang L, Guo G, Xu J, Sun X, Chen W, Jin J et al (2017) Human cytomegalovirus detection in gastric cancer and its possible association with lymphatic metastasis. Diagn Microbiol Infect Dis 88:62–68
Chan G, Nogalski MT, Yurochko AD (2009) Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. PNAS Natl Acad Sci 106:22369–22374
Wu Y, Prager A, Boos S, Resch M, Brizic I, Mach M et al (2017) Human cytomegalovirus glycoprotein complex gH/gL/gO uses PDGFR-α as a key for entry. PLoS Pathog 13:e1006281
Mohebbi A, Mamizadeh Z, Bagheri H, Sharifnezhad F, Tabarraei A, Yazdi M (2020) Prevalent latent human cytomegalovirus genotype b2 in biopsy samples of gastric cancer. Future Virol Future Med 15:71–78
Fattahi S, Kosari-Monfared M, Ghadami E, Golpour M, Khodadadi P, Ghasemiyan M et al (2018) Infection-associated epigenetic alterations in gastric cancer: new insight in cancer therapy. J Cell Physiol 233:9261–9270
Kosari-Monfared M, Nikbakhsh N, Fattahi S, Ghadami E, Ranaei M, Taheri H et al (2019) CTNNBIP1 downregulation is associated with tumor grade and viral infections in gastric adenocarcinoma. J Cell Physiol 234:2895–2904
Shi L, Fan B, Chen D, Guo C, Xiang H, Nie Y et al (2020) Human cytomegalovirus protein UL136 activates the IL-6, STAT3 signal through MiR-138 and MiR-34c in gastric cancer cells. Int J Clin Oncol. https://doi.org/10.1007/s10147-020-01749-z
Chen W, Lin K, Zhang L, Guo G, Sun X, Chen J et al (2015) The cytomegalovirus protein UL138 induces apoptosis of gastric cancer cells by binding to heat shock protein 70. Oncotarget Impact J 7:5630–5645
Meng L, Ding L, Yu Y, Li W (2020) JAK3 and TYK2 serve as prognostic biomarkers and are associated with immune infiltration in stomach adenocarcinoma. BioMed Res Int. https://doi.org/10.1155/2020/7973568
Wöss K, Simonović N, Strobl B, Macho-Maschler S, Müller M (2019) TYK2: an upstream kinase of STATs in cancer. Cancers 11:1728
Yap TA, Bjerke L, Clarke PA, Workman P (2015) Drugging PI3K in cancer: refining targets and therapeutic strategies. Curr Opin Pharmacol 23:98–107
Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D et al (2007) Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer 120:1803–1810
Genentech, Inc (2020) A randomized, phase II, placebo-controlled study of Ipatasertib (GDC-0068), an inhibitor to Akt, in combination with fluoropyrimidine plus oxaliplatin in patients with locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma. clinicaltrials.gov. Report No.: NCT01896531. https://clinicaltrials.gov/ct2/show/NCT01896531
Novartis Pharmaceuticals (2020) A phase IB, multicenter, open-label dose escalation study of the PI3K inhibitor BYL719 in combination with the HSP90 inhibitor AUY922 in patients with advanced or metastatic gastric cancer carrying a molecular alteration of PIK3CA or an amplification of HER2. clinicaltrials.gov. Report No.: NCT01613950. https://clinicaltrials.gov/ct2/show/NCT01613950
Ang YLE, Yong WP, Tan P (2016) Translating gastric cancer genomics into targeted therapies. Crit Rev Oncol Hematol 100:141–146
Amgen (2016) A Phase 1, first-in-human study evaluating the safety, tolerability, and pharmacokinetics of AMG 337 in adult subjects with advanced solid tumors. clinicaltrials.gov. Report No.: NCT01253707. https://clinicaltrials.gov/ct2/show/NCT01253707
Amgen (2017) A multicenter, Phase 2, single arm, two cohort study evaluating the efficacy, safety, and pharmacokinetics of AMG337 in subjects with MET amplified gastric/gastroesophageal junction/esophageal adenocarcinoma or other MET amplified solid tumors. clinicaltrials.gov. Report No.: NCT02016534. https://clinicaltrials.gov/ct2/show/NCT02016534
C-Met (2020) Inhibitor AMG 337, oxaliplatin, leucovorin calcium, and fluorouracil in treating patients with advanced stomach or esophageal cancer—full text view. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02344810
Magnelli L, Schiavone N, Staderini F, Biagioni A, Papucci L (2020) MAP kinases pathways in gastric cancer. Int J Mol Sci 21:2893
Cui Y, Yu S, Zhu M, Cheng X, Yu Y, Tang Z et al (2020) Identifying predictive factors of recurrence after radical resection in gastric cancer by RNA immune-oncology panel. J Cancer 11:638–647
Pottier C, Fresnais M, Gilon M, Jérusalem G, Longuespée R, Sounni NE (2020) Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers 12:731
Fontana E, Smyth EC (2016) Novel targets in the treatment of advanced gastric cancer: a perspective review. Ther Adv Med Oncol 8:113–125
Lin W, Kao H-W, Robinson D, Kung H-J, Wu C-W, Chen H-C (2000) Tyrosine kinases and gastric cancer. Oncogene 19:5680–5689
Nelson EA, Walker SR, Kepich A, Gashin LB, Hideshima T, Ikeda H et al (2008) Nifuroxazide inhibits survival of multiple myeloma cells by directly inhibiting STAT3. Blood 112:5095–5102
Sunakawa Y, Lenz H-J (2015) Molecular classification of gastric adenocarcinoma: translating new insights from the cancer genome atlas research network. Curr Treat Options in Oncol 16:17
Li S, Du H, Wang Z, Zhou L, Zhao X, Zeng Y (2010) Meta-analysis of the relationship between Epstein–Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci China Life Sci 53:524–530
Gryko M, Pryczynicz A, Zareba K, Kędra B, Kemona A, Guzińska-Ustymowicz K (2014) The expression of Bcl-2 and BID in gastric cancer cells. J Immunol Res 2014:e953203
Hirata Y, Maeda S, Mitsuno Y, Akanuma M, Yamaji Y, Ogura K et al (2001) Helicobacter pylori activates the cyclin D1 gene through mitogen-activated protein kinase pathway in gastric cancer cells. Infect Immun 69:3965–3971
Yau TO, Tang C-M, Yu J (2014) Epigenetic dysregulation in Epstein-Barr virus-associated gastric carcinoma: disease and treatments. World J Gastroenterol 20:6448–6456
Judd LM, Menheniott TR, Ling H, Jackson CB, Howlett M, Kalantzis A et al (2014) Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS ONE 9:e95993
Schönrich G, Raftery MJ (2019) The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol 9:207
Wang X, Zhang Y, Jiang L, Zhou F, Zhai H, Zhang M et al (2016) Interpreting the distinct and shared genetic characteristics between Epstein-Barr virus associated and non-associated gastric carcinoma. Gene 576:798–806
Altman AM, Mahmud J, Nikolovska-Coleska Z, Chan G (2019) HCMV modulation of cellular PI3K/AKT/mTOR signaling: new opportunities for therapeutic intervention? Antiviral Res 163:82–90
Servetas SL, Bridge DR, Merrell DS (2016) Molecular mechanisms of gastric cancer initiation and progression by Helicobacter pylori. Curr Opin Infect Dis 29:304–310
Zhao J, Liang Q, Cheung K-F, Kang W, Lung RWM, Tong JHM et al (2013) Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells: EBV-driven methylation in gastric cancer. Cancer 119:304–312
Baud J, Varon C, Chabas S, Chambonnier L, Darfeuille F, Staedel C (2013) Helicobacter pylori initiates a mesenchymal transition through ZEB1 in gastric epithelial cells. PLoS ONE 8:e60315
Teo WH, Chen H-P, Huang JC, Chan Y-J (2017) Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells. Int J Oncol 51:1415–1426
Voigtlaender M, Schneider-Merck T, Trepel M (2018) Lapatinib. In: Martens UM (ed) Small molecules in oncology. Springer International Publishing, Cham, pp 19–44
Dennie TW, Fleming RA, Bowen CJ et al (2011) A phase I study of capecitabine, oxaliplatin, and lapatinib in metastatic or advanced solid tumors. Clin Colorectal Cancer 10:57–62
Croxtall JD, McKeage K (2010) Trastuzumab: in HER2-positive metastatic gastric cancer. Drugs 70:2259–2267
Katoh M (2016) FGFR inhibitors: effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int J Mol Med 38:3–15
Xie L, Su X, Zhang L, Yin X, Tang L, Zhang X et al (2013) FGFR2 gene amplification in gastric cancer predicts sensitivity to the selective FGFR inhibitor AZD4547. Clin Cancer Res 19:2572–2583
Deng N, Goh LK, Wang H, Das K, Tao J, Tan IB et al (2012) A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 61:673–684
Peng R, Chen Y, Wei L et al (2020) Resistance to FGFR1-targeted therapy leads to autophagy via TAK1/AMPK activation in gastric cancer. Gastric Cancer 23:988–1002
Navas T, Kinders RJ, Lawrence SM et al (2020) Clinical evolution of epithelial-mesenchymal transition in human carcinomas. Cancer Res 80:304–318
Bhullar KS, Lagarón NO, McGowan EM, Parmar I, Jha A, Hubbard BP et al (2018) Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer BioMed Central 17:1–20
Vennepureddy A, Singh P, Rastogi R, Atallah J, Terjanian T (2017) Evolution of ramucirumab in the treatment of cancer: a review of literature. J Oncol Pharm Pract 23:525–539
Acknowledgements
This project was supported by Council of Scientific and Industrial Research Grant no. 37(1693)/17/EMR-II and Department of Science and Technology as Ramanujan fellowship grant no. SB/S2/RJN-132/20/5. DST-EMR: EMR/2017/001637.We are thankful to the Council of Scientific and Industrial Research and DST-Inspire for fellowship to Charu Sonkar and Nidhi Varshney respectively. The funding organization has not played any role in the study design or preparation of the manuscript. We appreciate our lab colleagues for insightful discussions and advice. We gratefully acknowledge the Indian Institute of Technology Indore for providing facilities and support. We further gratefully acknowledge Dr. Fabiola Ribeiro of Department of Biochemistry and Immunology, UFMG, Brazil for her efforts in correcting the language of the manuscript.
Funding
This project was supported by Council of Scientific and Industrial Research Grant no. 37(1693)/17/EMR-II and Department of Science and Technology as Ramanujan fellowship grant no. SB/S2/RJN-132/20/5. DST-EMR: EMR/2017/001637. We are thankful to the Council of Scientific and Industrial Research and DST-Inspire for fellowship to Charu Sonkar and Nidhi Varshney respectively. The funding organization has not played any role in the study design or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception or design of the work.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no competing interest.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sonkar, C., Varshney, N., Koganti, S. et al. Kinases and therapeutics in pathogen mediated gastric cancer. Mol Biol Rep 49, 2519–2530 (2022). https://doi.org/10.1007/s11033-021-07063-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-07063-9