Abstract
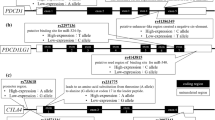
Toll-like receptors (TLRs) are a family of transmembrane receptors whose signaling control cellular processes of cell proliferation, survival, apoptosis, angiogenesis, remodeling, and repair of tissues. Polymorphisms in TLR genes can change the balance between pro and anti-inflammatory cytokines, modulating the risk of infection, chronic inflammation, and cancer. Although many studies have demonstrated the direct involvement of TLR signaling in the benefit of tumor cells in certain cancers, little is known about the influence of these gene polymorphisms on myeloproliferative neoplasms (MPNs). In this context, the objective of the study was to investigate a possible association between the TLR polymorphisms and the development of MPNs. 167 patients diagnosed with MPN and 222 healthy controls from the same region were evaluated. Genomic DNA was extracted and the TLR2 (rs5743708), TLR4 (rs4986790, rs4986791), TLR9 (rs5743836, rs187084) and JAK2V617F polymorphisms were genotyped by PCR-RFLP. The statistical analysis was performed by OpenEpi and SNPstat software. The JAK2V617F mutation was found in 68.32% of patients. TLR9-1486C/T CT genotype was less frequent in patients with polycythemia vera (PV) (OR 0.39, 95% CI 0.20–0.78, P = 0.025). When haplotype frequencies were analyzed, -1237T/-1486C (TLR9) was also less frequent in men (OR 0.58, 95% CI 0.36–0.94) and JAK negative men patients (OR 0.43, 95% CI 0.21–0.88). We can infer that the TLR9-1486 CT genotype could be associated with protection for PV and the TLR9-1237T/-1486C haplotype, protection for men, as well as for JAK negative men patients with MPN. There were no associations between TLR2 and TLR4 gene polymorphisms and MPN.
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tefferi A (2013) Primary myelofibrosis: 2013 update on diagnosis, risk stratification, and management. Am J Hematol 88(2):141–150. https://doi.org/10.1002/ajh.23384
Spivak JL, Barosi G, Tognoni G, Barbui T, Finazzi G, Marchioli R, Marchetti M (2003) Chronic myeloproliferative disorders. Hematology Am Soc Hematol Educ Program. https://doi.org/10.1182/asheducation-2003.1.200
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. https://doi.org/10.1038/nature01322
Drexler SK, Foxwell BM (2010) The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol 42(4):506–518. https://doi.org/10.1016/j.biocel.2009.10.009
Akira S, Takeda K (2004) Toll-like receptor signaling. Nat Rev Immunol 4(7):499–511. https://doi.org/10.1038/nri1391
Sfondrini L, Rossini A, Besusso D, Merlo A, Tagliabue E, Mènard S, Balsari A (2006) Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J Immunol 176:6624–6630. https://doi.org/10.4049/jimmunol.176.11.6624
Chang YJ, Wu MS, Lin JT, Chen CC (2005) Helicobacter pylori induced invasion and angiogenesis of gastric cells is mediated by cyclooxygenase-2 induction through TLR2/TLR9 and promoter regulation. J Immunol 175:8242–8252. https://doi.org/10.4049/jimmunol.175.12.8242
Bottero V, Busuttil V, Loubat A, Magné N, Fischel JL, Milano G, Peyron JF (2001) Activation of nuclear factor kappaB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res 61:7785–7791
Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J (2003) Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med 197:403–411. https://doi.org/10.1084/jem.20021633
Chochi K, Ichikura T, Kinoshita M, Majima T, Shinomiya N, Tsujimoto H, Kawabata T, Sugasawa H, Ono S, Seki S, Mochizukiet H (2008) Helicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cells. Clin Cancer Res 14:2909–2917
Jego G, Bataille R, Geffroy-Luseau A, Descamps G, PellatDeceunynck C (2006) Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia 20:1130–1137. https://doi.org/10.1038/sj.leu.2404226
Pries R, Hogrefe L, Xie L, Frenzel H, Brocks C, Ditz C, Wollenberg B (2008) Induction of c-Myc-dependent cell proliferation through toll-like receptor 3 in head and neck cancer. Int J Mol Med 21:209–215
Yu L, Chen S (2008) Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother 57(9):1271–1278. https://doi.org/10.1007/s00262-008-0459-8
Kutikhin AG (2011) Impact of toll-like receptor 4 polymorphisms on risk of cancer. Hum Immunol 72:193–206. https://doi.org/10.1016/j.humimm.2010.11.003
Texereau J, Chiche JD, Taylor W, Choukroun G, Comba B, Mira JP (2005) The importance of toll-Like receptor 2 polymorphisms in severe infections. Clin Infect Dis 41:408–415. https://doi.org/10.1086/431990
Rallabhandi P, Bell J, Boukhvalova MS, Medvedev A, Lorenz E, Arditi M, Hemming VG, Blanco JC, Segal DM, Vogel SN (2006) Analysis of TLR4 polymorphic variants: new insights into TLR4/MD-2/CD14 stoichiometry, structure, and signaling. J Immunol 177(1):322–332. https://doi.org/10.4049/jimmunol.177.1.322
Ferwerda B, McCall MBB, Verheijen K, Kullberg B, van der Ven AJ, Van der Meer JW, Netea MG (2008) Functional consequences of toll-like receptor 4 polymorphisms. Mol Med 14:346–352. https://doi.org/10.2119/2007-00135.Ferwerda
Ng MT, Hof RV, Crockett JC, Hope ME, Berry S, Thomson J, McLean MH, McColl KE, El-Omar EM, Hold GL (2010) Increase in NF-kappa B binding affinity of the variant C allele of the toll-like receptor 9-1237T/C polymorphism is associated with Helicobacter pylori-induced gastric disease. Infect Immun 78:1345–1352. https://doi.org/10.1128/IAI.01226-09
Tefferi A, Thiele J, Vardiman JW (2009) The 2008 World Health Organization classification system for myeloproliferative neoplasms: order out of chaos. Cancer 115(17):3842–3847. https://doi.org/10.1002/cncr.24440
Monte-Mór BCR, Cunha AF, Pagnano KBB, Saad ST, Lorand-Metze I, Costa FF (2007) JAK2 V617F prevalence in Brazilian patients with polycythemia vera, idiopathic myelofibrosis and essential thrombocythemia. Genet Mol Biol 30(2):336–338. https://doi.org/10.1590/S1415-47572007000300006
Folwaczny M, Glas J, Török HP, Limbersky O, Folwaczny C (2004) Toll-like receptor (TLR) 2 and 4 mutations in periodontal disease. Clin Exp Immunol 135(2):330–335. https://doi.org/10.1111/j.1365-2249.2004.02383.x
Selvaraj P, Harishankar M, Singh B, Jawahar MS, Banurekha VV (2010) Toll-like receptor and TIRAP gene polymorphisms in pulmonary tuberculosis patients of South India. Tuberculosis 90(5):306–310. https://doi.org/10.1016/j.tube.2010.08.001
Solé X, Guinó E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22(15):1928–1929. https://doi.org/10.1093/bioinformatics/btl268
Sullivan KM, Dean A, Soe MM (2009) OpenEpi: a web-based epidemiologic and statistical calculator for public health. Public Health Rep 124(3):471–474. https://doi.org/10.1177/003335490912400320
Ortmann CA, Kent DG, Nangalia J, Silber Y, Wedge DC, Grinfeld J, Baxter EJ, Massie CE, Papaemmanuil E, Menon S, Godfrey AL, Dimitropoulou D, Guglielmelli P, Bellosillo B, Besses C, Döhner K, Harrison CN, Vassiliou GS, Vannucchi A, Campbell PJ, Green AR (2015) Effect of mutation order on myeloproliferative neoplasms. N Engl J Med 372:601–612. https://doi.org/10.1056/NEJMoa1412098
Tao K, Fujii M, Tsukumo S, Maekawa Y, Kishihara K, Kimoto Y, Horiuchi T, Hisaeda H, Akira S, Kagami S, Yasutomo K (2007) Genetic variations of toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann Rheum Dis 66(7):905–909. https://doi.org/10.1136/ard.2006.065961
Pradere J-P, Dapito DH, Schwabe RF (2014) The Yin and Yang of toll-like receptors in cancer. Oncogene 33(27):3485–3495. https://doi.org/10.1038/onc.2013.302
Rahman HAA, Khorshied MM, Khorshid OMR, Mahgoub SM (2014) Toll-like receptor 2 and 9 genetic polymorphisms and the susceptibility to B cell non-Hodgkin lymphoma in Egypt. Ann Hematol 93:1859–1865. https://doi.org/10.1007/s00277-014-2131-z
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J (2008) WHO classification of tumours of haematopoietic and lymphoid tissues, 4th edn. International Agency for Research on Cancer, Lyon, p 439
Trifa AP, Cucuianu A, Petrov L, Urian L, Militaru MS, Dima D, Pop IV, Popp RA (2010) The G allele of the JAK2 rs10974944 SNP, part of the JAK2 46/1 haplotype, is strongly associated with JAK2 V617F-positive myeloproliferative neoplasms. Ann Hematol 89(10):979–983. https://doi.org/10.1007/s00277-010-0960-y
Pagliarini-e-Silva S, Santos BC, Pereira EM, Ferreira ME, Baraldi EC, Sell AM, Visentainer JEL (2013) Evaluation of the association between the JAK2 46/1 haplotype and chronic myeloproliferative neoplasms in a Brazilian population. Clinics 68(1):5–9. https://doi.org/10.6061/clinics/2013(01)oa02
Balistreri CR, Caruso C, Carruba G, Miceli V, Campisi I, Listì F, Lio D, Colonna-Romano G, Candore G (2010) A pilot study on prostate cancer risk and pro-inflammatory genotypes: pathophysiology and therapeutic implications. Curr Pharm Des 16:718–724. https://doi.org/10.2174/138161210790883877
Ashton KA, Proietto A, Otton G, Symonds I, McEvoy M, Attia J, Scott RJ (2010) Toll-like receptor (TLR) and nucleosome-binding oligomerization domain (NOD) gene polymorphisms and endometrial cancer risk. BMC Cancer 10:382. https://doi.org/10.1186/1471-2407-10-382
Boraska Jelavić T, Barisić M, Drmic Hofman I, Boraska V, Vrdoljak E, Peruzović M, Hozo I, Puljiz Z, Terzić J (2006) Microsatelite GT polymorphism in the toll-like receptor 2 is associated with colorectal cancer. Clin Genet 70:156–160. https://doi.org/10.1111/j.1399-0004.2006.00651.x
Kutikhin AG (2011) Association of polymorphisms in TLR genes and in genes of the toll-like receptor signaling pathway with cancer risk. Hum Immunol 72(11):1095–1116. https://doi.org/10.1016/j.humimm.2011.07.307
Acknowledgements
The authors thank to the collaborators of the LIG-UEM, Elo Integral Oncology/Maringa, Maringa Cancer Hospital, Maringa Regional Blood Center, Londrina Cancer Institute and Laboratory of Molecular Diagnosis of Hematologic Diseases at UNICAMP.
Funding
Conselho Nacional de Desenvolvimento Científico Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Laboratório de Imunogenética da UEM (Proc. No. 1589/2017-CSD-UEM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This work was approved and conducted according to the norms recommended by the Ethics Committee on Human Research of the State University of Maringa (CAAE: 14508313.2.0000.0104).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Quirino, M.G., Macedo, L.C., Pagnano, K.B.B. et al. Toll-like receptor gene polymorphisms in patients with myeloproliferative neoplasms. Mol Biol Rep 48, 4995–5001 (2021). https://doi.org/10.1007/s11033-021-06238-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06238-8