Abstract
This study was conducted to present the mechanism of the therapeutic effects of Chlorella vulgaris extract (CV) on the carbon tetrachloride (CCl4) induced liver fibrosis model. Primarily, the mechanism of antioxidant effects of CV were investigated via measuring the expression of forkhead box protein O1 (FOXO1) and phosphorylated 5′ adenosine monophosphate-activated protein kinase (p-AMPK) as upstream regulators of superoxide dismutase (SOD) and catalase (CAT). Subsequently, we investigated the regulatory effect of CV treatment on the yes-associated protein (YAP) and transcriptional coactivators with a PDZ-binding motif (TAZ) as fibrogenic factors. Male Wistar rats received CCl4 and olive oil solution 1 ml/kg intraperitoneally for 12 weeks, twice weekly. CV 50 and 100 mg/kg were administered on a daily basis by gavage in the last 4 weeks. Ultimately, liver marker enzymes and hepatic hydroxyproline content were measured. The activity of SOD and CAT and the expression of YAP, TAZ, FOXO1, SOD, and CAT were analyzed. Finally, the protein levels of YAP, TAZ, and p-AMPK were detected. CV administration decreased liver marker enzymes and hydroxyproline content significantly. The expression and protein levels of YAP and TAZ reduced by CV treatment. Furthermore, the augmentation of expression and function of CAT and SOD by CV treatment was followed by an increase in the expression of FOXO1 and protein level of p-AMPK. Our data revealed that the stimulation of expression and function of SOD and CAT by CV treatment could be mediated by FOXO1/p-AMPK axis. Moreover, anti-fibrotic effect of CV might be associated with its inhibitory effect on the hepatic expression of YAP and TAZ.
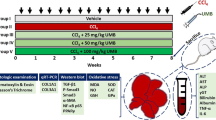
Graphic Abstract
Chlorella vulgaris treatment ameliorates liver fibrosis via two cellular mechanisms. A) Likely, Chlorella vulgaris treatment increases gene expression of enzymatic antioxidants superoxide dismutase (SOD) and catalase (CAT) via upregulating its upstream regulatory elements i.e. phosphorylated 5′ adenosine monophosphate-activated protein kinase (p-AMPK) and forkhead box protein O1 (FOXO1). These possible regulatory effects maybe lead to reduce reactive oxygen species level (ROS). B) Chlorella vulgaris treatment decreases hepatic protein level and gene expression of key elements of Hippo signaling pathway i.e. Yes-associated protein (YAP) and Transcriptional coactivators with a PDZ-binding motif (TAZ). Figure created with BioRender (https://biorender.com). ROS: Reactive oxygen species, YAP: Yes-associated protein, TAZ: Transcriptional coactivators with a PDZ-binding motif, FOXO1: Fork head Box O1, AMPK: 5′ adenosine monophosphate activated protein kinase, SOD: Superoxide dismutase, CAT: Catalase, P: Phosphate group.





Similar content being viewed by others
References
Brattin WJ, Glende EA Jr, Recknagel RO (1985) Pathological mechanisms in carbon tetrachloride hepatotoxicity. J Free Radic Biol Med 1(1):27–38
Clawson GA (1989) Mechanisms of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res 8(2):104–112
Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. J Hepatol 70(1):151–171
Xu F, Liu C, Zhou D, Zhang L (2016) TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem 64(3):157–167
Seki E, Brenner DA (2015) Recent advancement of molecular mechanisms of liver fibrosis. J Hepato-Biliary-Pancreat Sci 22(7):512–518
Kim C-L, Choi S-H, Mo J-S (2019) Role of the Hippo pathway in fibrosis and cancer. Cells 8(5):468
Wang X, Zheng Z, Caviglia JM, Corey KE, Herfel TM, Cai B, Masia R, Chung RT, Lefkowitch JH, Schwabe RF (2016) Hepatocyte TAZ/WWTR1 promotes inflammation and fibrosis in nonalcoholic steatohepatitis. Cell Metab 24(6):848–862
Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, Hoorens A, Reynaert H, Halder G, van Grunsven LA (2015) The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 63(3):679–688
Piersma B, Bank RA, Boersema M (2015) Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med 2:59
Mohseni R, Karimi J, Tavilani H, Khodadadi I, Hashemnia M (2019) Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-β signaling pathways in carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol 41(1):163–171
Sadeghabadi ZA, Ziamajidi N, Abbasalipourkabir R, Mohseni R, Borzouei S (2019) Palmitate-induced IL6 expression ameliorated by chicoric acid through AMPK and SIRT1-mediated pathway in the PBMCs of newly diagnosed type 2 diabetes patients and healthy subjects. Cytokine 116:106–114
Ix JH, Sharma K (2010) Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. J Am Soc Nephrol 21(3):406–412
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY-J (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13(4):376–388
Sadeghabadi ZA, Nourbakhsh M, Pasalar P, Emamgholipour S, Golestani A, Larijani B, Razzaghy-Azar M (2018) Reduced gene expression of sirtuins and active AMPK levels in children and adolescents with obesity and insulin resistance. Obes Res Clin Pract 12(2):167–173
Yun H, Park S, Kim MJ, Yang WK, Im DU, Yang KR, Hong J, Choe W, Kang I, Kim SS (2014) AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J 281(19):4421–4438
Ramezani-Moghadam M, Wang J, Ho V, Iseli TJ, Alzahrani B, Xu A, Van der Poorten D, Qiao L, George J, Hebbard L (2015) Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion. J Biol Chem 290(9):5533–5542
Sun L-J, Yu J-W, Wan L, Zhang X-Y, Shi Y-G, Chen M-Y (2014) Endocannabinoid system activation contributes to glucose metabolism disorders of hepatocytes and promotes hepatitis C virus replication. Int J Infect Dis 23:75–81
Morais ADS, Abarca-Quinones J, Guigas B, Viollet B, Stärkel P, Horsmans Y, Leclercq IA (2010) Development of hepatic fibrosis occurs normally in AMPK-deficient mice. Clin Sci 118(6):411–420
Baghy K, Iozzo RV, Kovalszky I (2012) Decorin–TGFβ axis in hepatic fibrosis and cirrhosis. J Histochem Cytochem 60(4):262–268
Ravan AP, Goudarzi F, Rafieemehr H, Bahmani M, Rad F, Jafari M, Mahmoodi M (2019) Human umbilical cord-mesenchymal stem cells conditioned medium attenuates CCl4 induced chronic liver fibrosis. Toxin Rev 1–12
Lim JY, Oh MA, Kim WH, Sohn HY, Park SI (2012) AMP-activated protein kinase inhibits TGF-β-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J Cell Physiol 227(3):1081–1089
Yang Y, Zhao Z, Liu Y, Kang X, Zhang H, Meng M (2015) Suppression of oxidative stress and improvement of liver functions in mice by ursolic acid via LKB 1-AMP-activated protein kinase signaling. J Gastroenterol Hepatol 30(3):609–618
Karimi J, Mohammadalipour A, Sheikh N, Khodadadi I, Hashemnia M, Goudarzi F, Khanjarsim V, Solgi G, Hajilooi M, Bahabadi M (2020) Protective effects of combined Losartan and Nilotinib on carbon tetrachloride (CCl4)-induced liver fibrosis in rats. Drug Chem Toxicol 43(5):468–478
Barghi M, Ashrafi M, Aminlari M, Namazi F, Nazifi S (2019) The protective effect of Zataria multiflora Boiss essential oil on CCl4 induced liver fibrosis in rats. Drug Chem Toxicol 1–9
Ravan AP, Bahmani M, Basir HRG, Salehi I, Oshaghi EA (2017) Hepatoprotective effects of Vaccinium arctostaphylos against CCl4-induced acute liver injury in rats. J Basic Clin Physiol Pharmacol 28(5):463–471
Safi C, Zebib B, Merah O, Pontalier P-Y, Vaca-Garcia C (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sustain Energy Rev 35:265–278
Mohseni R, Arab Sadeghabadi Z, Karimi J, Gholami H, Ghasemi H, Ghadimipour HR, Kheiripour N (2019) Chlorella vulgaris supplementation attenuates the progression of liver fibrosis through targeting TGF-β-signaling pathway in the CCl4-induced liver fibrosis in rats. Toxin Rev 1–9
Azocar J, Diaz A (2013) Efficacy and safety of Chlorella supplementation in adults with chronic hepatitis C virus infection. World J Gastroenterol 19(7):1085
Ebrahimi-Mameghani M, Aliashrafi S, Javadzadeh Y, AsghariJafarabadi M (2014) The effect of Chlorella vulgaris supplementation on liver enzymes, serum glucose and lipid profile in patients with non-alcoholic fatty liver disease. Health Promot Perspect 4(1):107
Mohseni R, Arab Sadeghabadi Z, Goodarzi MT, Karimi J (2019) Co-administration of resveratrol and beta-aminopropionitrile attenuates liver fibrosis development via targeting lysyl oxidase in CCl4-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol 1–8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Reddy GK, Enwemeka CS (1996) A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 29(3):225–229
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196(2–3):143–151
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408
Li L, Li W, Kim Y-h, Lee YW (2013) Chlorella vulgaris extract ameliorates carbon tetrachloride-induced acute hepatic injury in mice. Exp Toxicol Pathol 65(1–2):73–80
Vijayavel K, Anbuselvam C, Balasubramanian M (2007) Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol Cell Biochem 303(1–2):39–44
Xin Z, Ma Z, Hu W, Jiang S, Yang Z, Li T, Chen F, Jia G, Yang Y (2018) FOXO1/3: potential suppressors of fibrosis. Ageing Res Rev 41:42–52
Pan X, Zhang Y, Kim H-G, Liangpunsakul S, Dong XC (2017) FOXO transcription factors protect against the diet-induced fatty liver disease. Sci Rep 7:44597
Jiang S, Li T, Yang Z, Yi W, Di S, Sun Y, Wang D, Yang Y (2017) AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res Rev 38:18–27
Kang JW, Hong JM, Lee SM (2016) Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res 60(4):383–393
Zhu H, Chai Y, Dong D, Zhang N, Liu W, Ma T, Wu R, Lv Y, Hu L (2018) AICAR-induced AMPK activation inhibits the noncanonical NF-B pathway to attenuate liver injury and fibrosis in BDL rats. Can J Gastroenterol Hepatol
Chen Y, Qing W, Sun M, Lv L, Guo D, Jiang Y (2015) Melatonin protects hepatocytes against bile acid-induced mitochondrial oxidative stress via the AMPK-SIRT3-SOD2 pathway. Free Radic Res 49(10):1275–1284
Luo W-m, Kong J, Gong Y, Liu X-q, Yang R-x, Zhao Y-x (2015) Tongxinluo protects against hypertensive kidney injury in spontaneously-hypertensive rats by inhibiting oxidative stress and activating forkhead Box O1 signaling. PLoS ONE 10(12):e0145130
Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q, Li J, Zhang Q, Gao Y, Gao L (2014) Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J Nutr Biochem 25(11):1207–1217
Hemalatha A, Girija K, Parthiban C, Saranya C, Anantharaman P (2013) Antioxidant properties and total phenolic content of a marine diatom Navicula clavata and green microalgae Chlorella marina and Dunaliella salina. Adv Appl Sci Res 4(5):151–157
Luangmonkong T, Suriguga S, Mutsaers HA, Groothuis GM, Olinga P, Boersema M (2018) Targeting oxidative stress for the treatment of liver fibrosis. In: Reviews of physiology, biochemistry and pharmacology, vol 175. Springer, pp 71–102
Zhang K, Chang Y, Shi Z, Han X, Han Y, Yao Q, Hu Z, Cui H, Zheng L, Han T (2016) ω-3 PUFAs ameliorate liver fibrosis and inhibit hepatic stellate cells proliferation and activation by promoting YAP/TAZ degradation. Sci Rep 6:30029
Acknowledgements
This work was supported by a grant from Baqiyatallah University of Medical Sciences (97000527).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohseni, R., Alavian, S.M., Sadeghabadi, Z.A. et al. Therapeutic effects of Chlorella vulgaris on carbon tetrachloride induced liver fibrosis by targeting Hippo signaling pathway and AMPK/FOXO1 axis. Mol Biol Rep 48, 117–126 (2021). https://doi.org/10.1007/s11033-020-05978-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05978-3