Abstract
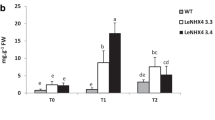
The function of the tomato K+, Na+/H+ antiporter LeNHX4 has been analyzed using 35S-driven gene construct for overexpressing a histagged LeNHX4 protein in Solanum lycopersicum L. Compared to wild-type plants, the expression of LeNHX4 was enhanced in most of plants transformed with a gene construct for LeNHX4 overexpression although some plants showed a decreased LeNHX4 expression. Overexpression of LeNHX4 was associated to an increased fruit size while silencing of this gene was related to a decreased fruit size. We have investigated the effect of LeNHX4 overexpression on fruit production and quality and we have also evaluated salt tolerance in two different overexpression lines by measuring proline, protein and glucose concentrations in tomato leaves grown either under control (0 mM NaCl) or saline (125 mM NaCl) conditions. Plants overexpressing LeNHX4 showed a higher amount of fruits than WT plants and accumulated higher contents of sugars and cations (Na+ and K+). The application of 125 mM NaCl, affected negatively fruit production and quality of WT plants. However the transgenic lines overexpressing LeNXH4 increased fruit quality and yield. In relation to salt tolerance, overexpression lines showed higher levels of leaf proline, glucose and proteins under NaCl treatment. The overexpression of LeNHX4 in tomato plants, improved salinity tolerance and increased fruit yield and quality under both normal and salinity stress conditions.




Similar content being viewed by others
References
FAO (2008) Land and plant nutrition management service. http://www.fao.org/ag/agl/agll/spush
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Kim JK, Bamba T, Harada K, Fukusaki E, Kobayashi A (2007) Time-course metabolic profiling in Arabidopsis thaliana cell cultures after salt stress treatment. J Exp Bot 58:415–424
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–668
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2012) Plant responses and tolerance to abiotic oxidative stress: antioxidant defenses is a key factors. In: Bandi V, Shanker AK, Shanker C, Mandapaka M (eds) Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–316
Osakabe Y, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS (2014) ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. N Phytol 202:35–49
Pettigrew WT (2008) Potassium influences on yield and quality production for maize, wheat, soybean and cotton. Physiol Plant 133:670–681
Miransari M, Smith DL (2007) Overcoming the stressful effects of salinity and acidity on soybean nodulation and yields using signal molecule genistein under field conditions. J Plant Nutr 30:1967–1992
Wakeel A, Farooq M, Qadir M, Schubert S (2011) Potassium substitution by sodium in plants. Crit Rev Plant Sci 30:401–413
Wakeel A (2013) Potassium–sodium interactions in soil and plant under saline-sodic conditions. J Plant Nutr Soil Sci 176:344–354
Serrano R, Gaxiola R (1994) Microbial models and salt stress tolerance in plants. Crit Rev Plant Sci 13:121–138
Hasegawa PM, Bresan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Baghour M, Gálvez FJ, Sánchez ME, Aranda MN, Venema K, Rodríguez-Rosales MP (2019) Overexpression of LeNHX2 and SlSOS2 increases salt tolerance and fruit production in double transgenic tomato plants. Plant Physiol Biochem 135:77–86
Cagnac O, Baghour M, Jaime-Pérez N, Aranda MN, Sánchez ME, Rodríguez-Rosales MP, Venema K (2020) Deletion of the N-terminal domain of the yeast vacuolar (Na+, K+)/H+ antiporter Vnx1p improves salt tolerance in yeast and transgenic Arabidopsis. Yeast 37:173–185
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Baghour M, Ben Chekroun K, Rodríguez-Rosales MP, Venema K (2010) Antiporters: role in salinity tolerance (a review). Moroc J Biol 6–7:16–22
Gálvez FJ, Baghour M, Hao G, Cagnac O, Rodríguez-Rosales MP, Venema K (2012) Expression of LeNHX isoforms in response to salt stress in salt sensitive and salt tolerant tomato species. Plant Physiol Biochem 51:109–115
Huang Z, Zhao L, Chen D, Liang M, Liu Z et al (2013) Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem Artichoke plantlets. PLoS ONE 8:e62085. https://doi.org/10.1371/journal.pone.0062085
Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4:401–406
Silva-Ortega CO, Ochoa-Alfaro AE, Reyes-Agüerob JA, Aguado-Santacruz GA, Jimenez-Bremont JF (2008) Salt stress increases the expression of P5CS gene and induces proline accumulation in Cactus pear. Plant Physiol Biochem 46:82–92
Moustakas M, Sperdouli I, Kouna T, Antonopoulou CI, Therios I (2011) Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul 65:315–325
Rasheed R, Wahid A, Farooq M, Hussain I, Basra SM (2011) Role of proline and glycine betaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp.) buds. Plant Growth Regul 65:35–45
Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102
Ma QJ, Sun MH, Lu J, Liu YJ, Hu DG, Hao YJ (2017) Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol 174:2348–2362
Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz T, Cubero B, Fernández JA, Bressan RA, Hasegawa PM, Quintero FJ, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61:495–506
Mishra S, Alavilli H, Lee B, Panda SK, Sahoo L (2014) Cloning and functional characterization of a vacuolar Na+/H+ antiporter gene from Mungbean (VrNHX1) and its ectopic expression enhanced salt tolerance in Arabidopsis thaliana. PLoS ONE 9:e106678. https://doi.org/10.1371/journal.pone.0106678
Mishra S, Alavilli H, Lee B, Panda SK, Sahoo L (2015) Cloning and characterization of a novel vacuolar Na+/H+ antiporter gene (VuNHX1) from drought hardy legume, cowpea for salt tolerance. Plant Cell Tissue Organ Cult 120:19–33
Metwali EMR, Soliman HIA, Fuller MP, Al-Zahrani HS, Howladar SM (2015) Molecular cloning and expression of a vacuolar Na+/H+, antiporter gene (AgNHX1) in fig (Ficus carica L.) under salt stress. Plant Cell Tissue Organ Cult 123(2):377–387
Pehlivan N, Sun L, Philip J, Yang X, Mishra N, Chen L, Kadioglu A, Shen G, Zhang H (2016) Co-overexpressing a plasma membrane and a vacuolar membrane sodium/proton antiporter significantly improves salt tolerance in transgenic Arabidopsis plants. Plant Cell Physiol 57:1069–1084
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) Binary vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Ellul P, García-Sogo B, Pineda B, Ríos G, Roig LA, Moreno V (2003) The ploidy level of transgenic plants in Agrobacterium-mediated transformation of tomato cotyledons (Lycopersicum esculentum Mill.) is genotype and procedure dependent. Theor Appl Genet 106:231–238
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acid Res 19:1349
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Circ 347:1–32
Schauer N, Zamir D, Fernie AR (2005) Metabolic profiling of leaves and fruit of wild species tomato: a survey of the Solanum lycopersicum complex. J Exp Bot 56:297–307
Irigoyen JJ, Emerich DW, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Paquin R, Lechasseur P (1979) Observations sur une méthode de dosage de la proline libre dans les extraits de plantes. Can J Bot 57:1851–1854
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Rodriguez-Rosales MP, Galvez FJ, Huertas R, Aranda MN, Baghour M, Cagnac O, Venema K (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4(4):265–276
Huertas R, Rubio L, Cagnac O, García-Sánchez MJ, Alché JD, Venema K, Fernández JA, Rodríguez-Rosales MP (2013) The K+/H+ antiporter LeNHX2 increases salt tolerance by improving K+ homeostasis in transgenic tomato. Plant Cell Environ 36:2135–2149
Huertas R, Olías R, Eljakaoui Z, Gálvez FJ, Li J, De Morales PA, Belver A, Rodríguez-Rosales MP (2012) Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ 35:1467–1482
Bassil E, Ohto M, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23:224–239
Li HT, Liu H, Gao XS, Zhang H (2009) Knock-out of Arabidopsis AtNHX4 gene enhances tolerance to salt stress. Biochem Biophys Res Commun 382:637–641
Liu H, Tang R, Zhang Y, Wang C, Lv Q, Gao X, Li W, Zhang H (2010) AtNHX3 is a vacuolar K+/H+ antiporter required for low-potassium tolerance in Arabidopsis thaliana. Plant Cell Environ 33:1989–1999
Sharma H, Taneja M, Upadhyay SK (2019) Identification, characterization and expression profiling of cation-proton antiporter superfamily in Triticum aestivum L. and functional analysis of TaNHX4-B. Genomics. https://doi.org/10.1016/j.ygeno.2019.02.015
Li N, Wang X, Ma B, Du C, Zheng L, Wang Y (2017) Expression of a Na+/H+ antiporter RtNHX1 from a recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana. J Plant Physiol 218:109–120
Reina-Sánchez A, Romero-Aranda R, Quartero J (2005) Plant water uptake and water use efficiency of greenhouse tomato cultivars irrigated with saline water. Agric Water Manag 78:54–66
Tal M, Katz A, Heikin H, Dehan K (1979) Salt tolerance in the wild relatives of the cultivated tomato: proline accumulation in Lycopersicon esculentum Mill., L. peruvianum Mill. and Solanum pennellii Cor. treated with NaCl and polyethyleneglycol. N Phytol 82:349–355
Ho LC, Grange RI, Picken AJ (1987) An analysis of the accumulation of water and dry matter in tomato fruit. Plant Cell Environ 10:157–162
Adams P (1991) Effects of increasing the salinity of the nutrient solution with major nutrients or sodium chloride on the yield, quality and composition of tomatoes grown in rockwool. J Hortic Sci 66:201–207
Balibrea M, Martinez-Andújar C, Cuartero J, Bolarín M, Pérez-Alfocea F (2006) The high fruit soluble sugar content in wild Lycopersicon species and their hybrids with cultivars depends on sucrose import during ripening rather than on sucrose metabolism. Funct Plant Biol 33:279–288
Gao Z, Sagi M, Lips SH (1998) Carbohydrate metabolism in leaves and assimilate partitioning in fruits of tomato (Lycopersicon esculentum L.) as affected by salinity. Plant Sci 135:149–159
Krauss SW, Schnitzler H, Grassmann J, Woitke M (2006) The influence of different electrical conductivity values in a simplified recalculating soilless system on inner and outer fruit quality characteristics of tomato. J Agric Food Chem 54:441–448
Saito T, Matsukura C, Ban Y, Shoji K, Sugiyama M, Fukuda N, Nishimura S (2008) Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J Jpn Soc Hortic Sci 77:61–68
Hanana M, Cagnac O, Zarrouk M, Blumwald E (2009) Rôles biologiques des antiports vacuolaires NHX : acquis et perspectives d’amélioration génétique des plantes. Botanique 87:1023–1035
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Rathinasabapathi B (2000) Metabolic engineering for stress tolerance: installing osmoprotectant synthesis pathways. Ann Bot 86:709–716
Liu H, Wang Q, Yu M, Zhang Y, Wu Y, Zhang H (2008) Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ 31:1325–1334
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Gharsallah C, Fakhfakh H, Grubb D, Gorsane F (2016) Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants. https://doi.org/10.1093/aobpla/plw055
Wang B, Zhai H, He S, Zhang H, Ren Z, Zhang D, Liu Q (2016) A vacuolar Na+/H+, antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweet potato. Sci Hortic 201:153–166
Mansour M (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Ashraf M, Harris PGC (2004) Biochemical indicators of salinity tolerance in plant. Plant Sci 166:3–16
Parvaiz A, Satyawati S (2008) Salt stress and phyto-biochemical responses of plants—a review. Plant Soil Environ 54:89–99
Veeranagamallaiah G, Chandraobulreddy P, Jyothsnakumari G, Sudhakar C (2007) Glutamine synthetase expression and pyrroline-5-carboxylate reductase activity influence proline accumulation in two cultivars of foxtail millet (Setaria italica L.) with differential salt sensitivity. Environ Exp Bot 60:239–244
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–507
Kahlaoui B, Hachicha M, Misle E, Fidalgo F, Teixeira J (2018) Physiological and biochemical responses to the exogenous application of proline of tomato plants. J Saudi Soc Agric Sci 17:17–23
Singh NK, Bracken CA, Hasegawa PM, Handa AK, Buckel S, Hermodson MA, Pfankoch F, Regnier FE, Bressan RA (1987) Characterization of osmotin. A thaumatin-like protein associated with osmotic adjustment in plant cells. Plant Physiol 85:529–536
Ashraf M, Fatima H (1995) Responses of some salt tolerant and salt sensitive lines of safflower (Carthamustin ctorius L.). Acta Physiol Plant 17:61–71
Acknowledgements
This work was supported by Grants from Consejería de Economía, Innovación, Ciencia y Empresa, Junta de Andalucía, Spain (CVI-7558 to MPRR), Spanish Ministry of Economy and Competitiveness and Agencia Estatal de Investigación (BIO2015-65056-P, BIO2016-81957-REDT/AEI and Programa I-COOPB+2013 Ref. COOPB20053). National Centre for Scientific and Technical Research and Minister for Higher Education, Scientific Research and Executive Training (Morocco).
Author information
Authors and Affiliations
Contributions
Study concepts: MB, KV, MPRR. Study design: MB, KV, MPRR. Literature research: MM, MB, MPRR. Experimental studies: MM, FJG, MES, MNA. Data analysis/interpretation: MM, MB, MA, FJG, KV, MPRR. Statistical analysis: MM, MB. Manuscript preparation: MM, MB, MPRR. Manuscript revision: MB, MPRR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript does not imply human participants or studies on animals.
Informed consent
Not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maach, M., Baghour, M., Akodad, M. et al. Overexpression of LeNHX4 improved yield, fruit quality and salt tolerance in tomato plants (Solanum lycopersicum L.). Mol Biol Rep 47, 4145–4153 (2020). https://doi.org/10.1007/s11033-020-05499-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05499-z