Abstract
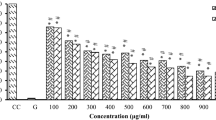
Pancreatic cancer (PC) is one of the leading causes of cancer death in Western societies. The absence of specific symptoms, late diagnosis and the resistance towards chemotherapy result in significant treatment difficulties. As such, it is important to find more effective therapeutic agents for the treatment of PC. Helicteres hirsuta Lour. (H. hirsuta) has been traditionally used in many countries for the treatment of various ailments, indicating that it contains potential therapeutic agents. This study aimed to derive different fractions from the saponin-enriched extract of H. hirsuta stem using RP-HPLC and examine the in vitro anti-pancreatic cancer activity of the derived fractions (F0–F5). With the exception of F0, the five fractions (F1–F5) possessed strong inhibitory activity against PC cells at IC50 values of 3.11–17.12 μg/mL. The flow cytometry assays revealed the active fractions caused cell cycle arrest at S phase and promoted apoptosis in MIAPaCa-2 PC cells. The LC/MS analysis revealed that the isolated fractions contained bioactive compounds, such as caffeic acid, rosmarinic acid, sagerinic acid, usnic acid, cucurbitacins and absinthin. It can be concluded that the fractions isolated from H. hirsuta stem exhibit potent in vitro anti-pancreatic cancer activity and thus warrant further in vivo studies to assess their activity against PC followed by isolation of individual bioactive compounds and the evaluation of their anti-pancreatic cancer activity.




Similar content being viewed by others
References
Yu Z, Zhang T, Zhou F, Xiao X, Ding X, He H, Rang J, Quan M, Wang T, Zuo M (2015) Anticancer activity of saponins from Allium chinense against the B16 melanoma and 4T1 breast carcinoma cell. Evid Based Complement Altern Med. https://doi.org/10.1155/2015/725023
Chen J, Peng H, Ou-Yang X, He X (2008) Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res 18:322–329. https://doi.org/10.1097/CMR.0b013e32830b3536
Pham HNT, Nguyen VT, Vuong QV, Bowyer MC, Scarlett CJ (2015) Effect of extraction solvents and drying methods on the physicochemical and antioxidant properties of Helicteres hirsuta Lour. leaves. Technologies 3:285–301. https://doi.org/10.3390/technologies3040285
Pham HNT, Nguyen VT, Vuong QV, Bowyer MC, Scarlett CJ (2017) Bioactive compound yield and antioxidant capacity of Helicteres hirsuta Lour. stem as affected by various solvents and drying methods. J Food Process Preserv 41:12879. https://doi.org/10.1111/jfpp.12879
Pham HNT, Sakoff JA, Bond DR, Vuong QV, Bowyer MC, Scarlett CJ (2018) In vitro antibacterial and anticancer properties of Helicteres hirsuta Lour. leaf and stem extracts and their fractions. Mol Biol Rep 45:2125–2133. https://doi.org/10.1007/s11033-018-4370-x
Pham HNT, Vuong QV, Bowyer MC, Scarlett CJ (2017) Optimization of ultrasound-assisted extraction of Helicteres hirsuta Lour. for enhanced total phenolic compound and antioxidant yield. J Appl Res Med Aromat Plants 7:113–123. https://doi.org/10.1016/j.jarmap.2017.07.002
Pham HNT, Vuong QV, Bowyer MC, Scarlett CJ (2017) Phytochemical profiles and antioxidant capacity of the crude extracts, aqueous- and saponin-enriched butanol fractions of Helicteres hirsuta Lour. leaves and stems. Chem Pap 71:2233–2242. https://doi.org/10.1007/s11696-017-0216-6
Bhuyan DJ, Vuong QV, Bond DR, Chalmers AC, Bowyer MC, Scarlett CJ (2018) Eucalyptus microcorys leaf extract derived HPLC-fraction reduces the viability of MIA PaCa-2 cells by inducing apoptosis and arresting cell cycle. Biomed Pharmacother 105:449–460. https://doi.org/10.1016/j.biopha.2018.05.150
Deer EL, González-Hernández J, Coursen JD, Shea JE, Ngatia J, Scaife CL, Firpo MA, Mulvihill SJ (2010) Phenotype and genotype of pancreatic cancer cell lines. Pancreas 39:425. https://doi.org/10.1097/MPA.0b013e3181c15963
Schwartz GK, Shah MA (2005) Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol 23:9408–9421. https://doi.org/10.1200/JCO.2005.01.5594
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24:1770–1783. https://doi.org/10.1200/JCO.2005.03.7689
Santos RC, Salvador JA, Cortés R, Pachón G, Marín S, Cascante M (2011) New betulinic acid derivatives induce potent and selective antiproliferative activity through cell cycle arrest at the S phase and caspase dependent apoptosis in human cancer cells. Biochimie 93:1065–1075. https://doi.org/10.1016/j.biochi.2011.02.014
Quang DN, Pham CT, Le LTK, Ta QN, Dang NK, Hoang NT, Pham DH (2018) Cytotoxic constituents from Helicteres hirsuta collected in Vietnam. Nat Prod Res. https://doi.org/10.1080/14786419.2018.1490907
Goldsmith CD, Bond DR, Jankowski H, Weidenhofer J, Stathopoulos CE, Roach PD, Scarlett CJ (2018) The olive biophenols oleuropein and hydroxytyrosol selectively reduce proliferation, influence the cell cycle, and induce apoptosis in pancreatic cancer cells. Int J Mol Sci 19:1937. https://doi.org/10.3390/ijms19071937
Arora S, Bhardwaj A, Srivastava SK, Singh S, McClellan S, Wang B, Singh AP (2011) Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS ONE 6:e21573. https://doi.org/10.1371/journal.pone.0021573
Gülçin İ (2006) Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 217:213–220. https://doi.org/10.1016/j.tox.2005.09.011
Swamy MK, Sinniah UR, Ghasemzadeh A (2018) Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl Microbiol Biotechnol 102:7775–7793. https://doi.org/10.1007/s00253-018-9223-y
Adomako-Bonsu AG, Chan SL, Pratten M, Fry JR (2017) Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: importance of physico-chemical characteristics. Toxicol In Vitro 40:248–255. https://doi.org/10.1016/j.tiv.2017.01.016
Rahbardar MG, Amin B, Mehri S, Mirnajafi-Zadeh SJ, Hosseinzadeh H (2017) Anti-inflammatory effects of ethanolic extract of Rosmarinus officinalis L. and rosmarinic acid in a rat model of neuropathic pain. Biomed Pharmacother 86:441–449. https://doi.org/10.1016/j.biopha.2016.12.049
Ranković B, Kosanić M, Stanojković T, Vasiljević P, Manojlović N (2012) Biological activities of Toninia candida and Usnea barbata together with their norstictic acid and usnic acid constituents. Int J Mol Sci 13:14707–14722. https://doi.org/10.3390/ijms131114707
Singh N, Nambiar D, Kale RK, Singh RP (2013) Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells. Nutr Cancer 65:36–43. https://doi.org/10.1080/01635581.2013.785007
Bačkorová M, Bačkor M, Mikeš J, Jendželovský R, Fedoročko P (2011) Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol In Vitro 25:37–44. https://doi.org/10.1016/j.tiv.2010.09.004
Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX (2005) Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep 22:386–399. https://doi.org/10.1039/B418841C
Lee DH, Iwanski GB, Thoennissen NH (2010) Cucurbitacin: ancient compound shedding new light on cancer treatment. Sci World J 10:413–418. https://doi.org/10.1100/tsw.2010.44
Bean MF, Antoun M, Abramson D, Chang C-J, McLaughlin JL, Cassady JM (1985) Cucurbitacin B and isocucurbitacin B: cytotoxic components of Helicteres isora. J Nat Prod 48:500–500. https://doi.org/10.1021/np50039a033
Acknowledgements
We are thankful to the University of Newcastle, Australia that awarded the scholarships to the first author. We greatly appreciate Nathan Smith from the Analytical & Biomolecular Research Facility, Research and Innovation Division - Central Scientific Services at the University of Newcastle, Australia for his assistance in performing LC/MS analysis of the sample. The first author would like to thank Dr Danielle R. Bond, Dr Joshua Brzozowski, Benjamin Munro, Melanie Predebon and Rebecca Richmond for their expertise, advice and support in the cell culture lab.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pham, H.N.T., Vuong, Q.V., Bowyer, M.C. et al. In vitro anti-pancreatic cancer activity of HPLC-derived fractions from Helicteres hirsuta Lour. stem. Mol Biol Rep 47, 897–905 (2020). https://doi.org/10.1007/s11033-019-05180-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05180-0