Abstract
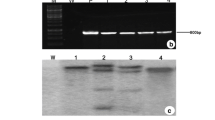
Sunflower (Helianthus annuus. L) is one of the principal oil seed crops affected by the salinity stress, which limits the oil content and crop yield of sunflower plants. The acclimatization of plants to abiotic stresses such as salinity tolerance is mainly mediated by the vacuolar Na+/H+ antiporters (NHX) by tagging Na+ into vacuoles from the cytosol. We show here that the over-expression of wheat TaNHX2 gene in transgenic sunflower conferred improved salinity stress tolerance and growth performance. Transgenic sunflower plants were produced by infecting the embryonic axis ex-plants with Agrobacterium tumefaciens strain EHA105 containing a pBin438-TaNHX2 binary vector that carried a wheat antiporter (TaNHX2) gene under the control of a double CaMV 35S promoter with NPT II gene as a selectable marker. PCR analysis of T0 and T1 transgenic plants confirmed the integration of TaNHX2 in sunflower genome. Stable integration and expression of TaNHX2 in sunflower genome was further verified by Southern hybridization and semi-quantitative RT-PCR analyses. As compared to the non-transformed plants, TaNHX2 expressing transgenic plants showed better growth performance and accumulated higher Na+, K+ contents in leaves and roots under salt stress (200 mM NaCl). Transgenic sunflower plants displayed improved protection against cell damage exhibiting stable relative water content, chlorophyll content, increased proline accumulation and improved reactive oxygen species (ROS) scavenging because of higher activities of the antioxidant enzymes like superoxide dismutase and ascorbate peroxidase, along with decreased production of hydrogen peroxide, free oxygen radical and malondialdehyde (MDA) under salt stress (200 mM NaCl). Taken together, our findings suggest that TaNHX2 expression in sunflower plants contributed towards improving growth performance under sodium chloride stress.






Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- DW:
-
Dry weight
- FW:
-
Fresh weight
- MDA:
-
Malondialdehyde
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- RWC:
-
Relative water content
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TW:
-
Fully turgid weight
References
Upadhyay SK, Singh DP (2015) Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol 17:288–293
Zhao Q, Zhang H, Wang T, Chen SX, Dai SJ (2013) Proteomics-based investigation of salt-responsive mechanisms in plant roots. J Proteomics 82:230–253
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10:615–620
Flowers TJ, Yeo AR (1995) Breeding for salinity resistance in crop plants: where next? Aust J Plant Physiol 22:875–884
Okorogbona AOM, Managa LR, Adebola PO, Ngobeni HM, Khosa TB (2015) Salinity and crop productivity. In: Lichtfouse E (ed) Sustainable agriculture reviews, 17. Springer, Cham
Vijayvargiya S, Kumar A (2011) Infuence of salinity stress on plant growth and productivity: salinity stress influences on plant growth. Lap Lambert Academic Publishers, Germany, p 170
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. Crit Rev Plant Sci 32:237–249
Ramankutty N, Mehrabi Z, Waha K, Jarvis L, Kremen C, Herreo M, Rieseberg LH (2018) Trends in global agriculturl land use: implications for environmental health and food safety. Ann Rev Plant Biol. https://doi.org/10.1146/annurev-arplant-042817-040256
Almeida DM, Oliveira MM, Saibo NJM (2017) Regulation of Na + and K+ homeostasis in plants: towards improved salt tolerance in crop plants. Genet Mol Biol 40:326–345
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Shi H, Lee BH, Wu SJ, Zhu JK (2002) Overexpression of a plasma membrane Na +//H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Blumwald E, Poole R (1985) Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol 78:163–167
Blumwald E, Poole R (1987) Salt-tolerance in suspension cultures of sugar beet. I. Induction of Na+/H+ antiport activity at the tonoplast by grown in salt. Plant Physiol 83:884–887
Yarra R (2019) The wheat NHX gene family: potential role in improving salinity stress tolerance of plants. Plant Gene 1:1. https://doi.org/10.1016/j.plgene.2019.100178
Liang W, Ma X, Wan P, Liu L (2018) Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun 495:286–291
Jiang X, Leidi EO, Pardo JM (2010) How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal Behav 55:792–795
Bassil E, Blumwald E (2014) The ins and outs of intracellular ion homeostasis: NHX-type cation/H+transporters. Curr Opin Plant Biol 22:1–6
Glenn EP, Brown JJ, Blumwald E (1999) Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci 18:227–256
Gaxiola RA, Rao R, Sherman A, Grisafi F, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNHX1 and AVP1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96:1480–1485
Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Fernández JA, Bressan RA, Hasegawa PM, Quintero FJ, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61:495–506
Reguera M, Bassil E, Blumwald E (2014) Intracellular NHX-type cation/H + antiporters in plants. Mol Plant 7:261–263
Bassil E, Coku A, Blumwald E (2012) Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot 63:5727–5740
Zhang H, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zeng Y, Li Q, Wang H, Zhang J, Du J, Feng H, Blumwald E, Yu L, Xu G (2018) Two NHX-type transporters from Helianthus tuberosus improve the tolerance of rice to salinity and nutrient deficiency stress. Plant Biotechnol J. https://doi.org/10.1111/pbi.12773
Huang Y, Guan C, Liu Y, Chen B, Yuan S, Cui X, Zhang Y, Yang F (2017) Enhanced growth performance and salinity tolerance in transgenic switchgrass via overexpressing vacuolar Na+ (K+)/H+ antiporter gene (PvNHX1). Front Plant Sci 8:458
Sahoo DP, Kumar S, Mishra S, Yasufumi Kobayashi Y, Panda SK, Sahoo L (2016) Enhanced salinity tolerance in transgenic mungbean overexpressing Arabidopsis antiporter (NHX1) gene. Mol Breed 36:144. https://doi.org/10.1007/s11032-016-0564-x
Pandey S, Patel MK, Mishra A, Jha B (2016) In planta transformed cumin (Cuminum cyminum L) plants, overexpressing the SbNHX1 gene showed enhanced salt endurance. PLoS ONE 11:e0159349. https://doi.org/10.1371/journal.pone.0159349
Fan W, Deng G, Wang H, Zhang H, Zhang P (2015) Elevated compartmentalization of Na+ into vacuoles improves salt and cold stress tolerance in sweet potato (Ipomoea batatas). Physiol Plant 154:560–571
Patel MK, Joshi M, Mishra A, Jha B (2015) Ectopic expression of SbNHX1 gene in transgenic castor (Ricinus communis L.) enhances salt stress by modulating physiological process. Plant Cell Tissue Organ Cult 122:477–490
Mishra S, Alavilli H, Lee BH, Panda SK, Sahoo L (2015) Cloning and characterization of a novel vacuolar Na+/H+ antiporter gene (VuNHX1) from drought hardy legume, cowpea for salt tolerance. Plant Cell Tissue Organ Cult 120:19–33
Mishra S, Behura R, Awasthi JP, Dey M, Sahoo DP, Bhowmik SSD, Panda SK, Sahoo L (2014) Ectopic overexpression of a mungbean vacuolar Na+/H+ antiporter gene (VrNHX1) leads to increased salinity stress tolerance in transgenic Vigna unguiculata L. Walp. Mol Breed 34:1345–1359
Jha B, Mishra A, Jha A, Joshi M (2013) Developing transgenic Jatropha using the SbNHX1 gene from an extreme halophyte for cultivation in saline wasteland. PLoS ONE 8:e71136. https://doi.org/10.1371/annotation/89bc2c6f-2799-4a5b-9f57-8e2fa3e14fc9
Banjara M, Zhu L, Shen G, Payton P, Zhang H (2012) Expression of an Arabidopsis sodium/proton antiporter gene (AtNHX1) in peanut to improve salt tolerance. Plant Biotechnol Rep 6:59–67
Bhaskaran S, Savithramma DL (2011) Co-expression of Pennisetum glaucum vacuolar Na+/H+ antiporter and Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic tomato. J Exp Bot 1:1. https://doi.org/10.1093/jxb/err237
Li W, Wang D, Jin T, Chang Q, Yin D, Xu S, Liu B, Liu L (2011) The vacuolar Na+/H+ antiporter gene SsNHX1 from the halophyte Salsola soda confers salt tolerance in transgenic alfalfa (Medicago sativa L.). Plant Mol Biol Rep 29:278–290
Zhang GH, Su Q, An LJ, Wu S (2008) Characterization and expression of a vacuolar Na+/H+ antiporter gene from the monocot halophyte Aeluropus littoralis. Plant Physiol Biochem 46:117–126
Chen LH, Zhang B, Xu ZQ (2008) Salt tolerance conferred by overexpression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 in common buckwheat (Fagopyrum esculentum). Transgenic Res 17:121–132
Rajagopal D, Agarwal P, Tyagi W, Singla-Pareek SL, Reddy MK, Sopory SK (2007) Pennisetum glaucum Na+/H+ antiporter confers high level of salinity tolerance in transgenic Brassica juncea. Mol Breed 19:137–151
Verma D, Singla-Pareek SL, Rajagopal D, Reddy MK, Sopory SK (2007) Functional validation of a novel isoform of Na+/H+ antiporter from Pennisetum glaucum for enhancing salinity tolerance in rice. J Biosci 32:621–628
Chen H, An R, Tang JH, Cui XH, Hao FS, Chen J, Wang CW (2007) Overexpression of a vacuolar Na+/H+ antiporter gene improves salt tolerance in an upland rice. Mol Breed 19:215–225
Brini F, Hanin M, Mezghani I, Berkowitz GA, Masmoudi K (2007) Overexpression of wheat Na+/H+ antiporter TNHX1 and Hþ-pyrophosphatase TVP1 improve salt- and drought-stress tolerance in Arabidopsis thaliana plants. J Exp Bot 58:301e–308e
Zhao J, Zhi D, Xue Z, Liu H, Xia G (2007) Enhanced salt tolerance of transgenic progeny of tall fescue (Festuca arundinacea) expressing a vacuolar Na+/H+ antiporter gene from Arabidopsis. J Plant Physiol 164:1377–1383
Hossain GS, Waditee R, Hibino T, Tanaka Y, Takabe T (2006) Root specific expression of Na+/H+ antiporter gene from Synechocystis sp.PCC6803 confers salt tolerance of tobacco plant. Plant Biotechnol 23:275–281
Zhao FY, Zhang XJ, Li PH, Zhao YX, Zhang H (2006) Coexpression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1. Mol Breed 17:341–353
Lu SY, Jing YX, Shen SH, Zhao HY, Ma LQ, Zhou XJ et al (2005) Antiporter gene from Hordum brevisubulatum (Trin.) link and its overexpression in transgenic tobaccos. J Integr Plant Biol 47:343–349
He C, Yan J, Shen G, Fu L, Holaday AS, Auld D, Blumwald E, Zhang H (2005) Expression of an Arabidopsis vacuolar sodium/proton antiporter gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the field. Plant Cell Physiol 46:1848–1854
Wu CA, Yang GD, Meng QW, Zheng CC (2004) The cotton GhNHX1 gene encoding a novel putative tonoplast Na+/H+ antiporter plays an important role in salt stress. Plant Cell Physiol 45:600–607
Zörb C, Noll A, Karl S, Leib K, Yan F, Schubert S (2004) Molecular characterization of Na+/H+ antiporters (ZmNHX) of maize (Zea mays L.) and their expression under salt stress. J Plant Physiol 162:55–66
Fukuda A, Nakamura A, Tageri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y (2004) Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol 45:146
Yin XY, Yang AF, Zhang KW, Zhang JR (2004) Production and analysis of transgenic maize with improved salt tolerance by the introduction of AtNHX1 gene. Acta Bot Sin 46:854–861
Xue ZY, Zhi DY, Xue GP, Zhang H, Zhao YX, Xia GM (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased sodium accumulation. Proc Natl Acad Sci USA 98:12832–12836
Brini F, Gaxiola RA, Berkowitz GA, Masmoudi K (2005) Cloning and characterization of a wheat vacuolar cation/proton antiporter and pyrophosphatase proton pump. Plant Physiol Biochem 43:347e–354e
Yu JN, Huang J, Wang ZN, Zhang JS, Chen SY (2007) An Na+/H+ antiporter gene from wheat plays an important role in stress tolerance. J Biosci 32:1153e–1161e
Lu W, Guo C, Li X, Duan W, Ma C, Zhao M, Gu J, Du X, Liu Z, Xiao K (2014) Overexpression of TaNHX3, a vacuolar Na+/H+ antiporter gene in wheat, enhances salt stress tolerance in tobacco by improving related physiological processes. Plant Physiol Biochem 76:17–28
Yarra R, Kirti PB (2019) Expressing class I wheat NHX (TaNHX2) gene in eggplant (Solanum melongena L.) improves plant performance under saline condition. Funct Integr Genomics 1:1. https://doi.org/10.1007/s10142-019-00656-5
Bulle M, Yarra R, Abbagani S (2016) Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol Breed 36:36. https://doi.org/10.1007/s11032-016-0451-5
Yarra R, He SJ, Abbagani S, Ma B, Bulle M, Zhang WK (2012) Overexpression of a wheat Na+/H+ antiporter gene (TaNHX2) enhances tolerance to salt stress in transgenic tomato plants (Solanum lycopersicum L.). Plant Cell Tissue Organ Cult 111:49–57
Zhang YM, Liu ZH, Wen ZY, Zhang HM, Yang F, Guo XL (2012) The vacuolar Na+/H+ antiport gene TaNHX2 confers salt tolerance on transgenic alfalfa (Medicago sativa). Funct Plant Biol 39:708–716
Wu LM, Chen W, Zhao Y, Feng SG, Ying QC, Liu JJ, Wang HZ (2012) Salt tolerance enhancement of transgenic rice with Na+/H+ antiporter gene driven by root specific promoter PmPgPR10. China J Rice Sci 26:643–650
Cao D, Hou W, Liu W, Yao WW, Wu C, Liu X, Han T (2011) Overexpression of TaNHX2 enhances salt tolerance of ‘composite’ and whole transgenic soybean plants. Plant cell Tiss Organ Cult. https://doi.org/10.1007/s11240-011-0005-9
Rauf S, Jamil N, Ali Tariq S, Khan M, Kausar M (2017) Progress in modification of sunflower oil to expand its industrial value. J Sci Food Agric 97:1997–2006
Dimitrijevic A, Imerovski I, Miladinovic D, Cvejic S, Jocic S, Zeremski T et al (2017) Oleic acid variation and marker-assisted detection of Pervenets mutation in high- and low-oleic sunflower cross. Crop Breed Appl Biotechnol 17:229–235
Caterina RD, Giuliani MM, Rotunno T, Caro AD, Flagella Z (2007) Influence of salt stress on seed yield and oil quality of two sunflower hybrids. Ann Appl Biol 151:145–154
Hardwick, R., & Ferguson, D. (1978). Germination and hypocotyl growth in Helianthus annuus L. as influenced by differential salt treatments. In: Procedings of the 8th International Sunflower Conference, Minneapolis, MN, 23–27 July. International Sunflower Associatiion, Paris, France, pp 385–399
Heikal MM, Ahmad AM, Shaddad A (1980) Changes in dry weight and mineral composition of some oil producing plants over a range of salinity stresses. Biol Plant 22:25–33
Blamey FPC, Asher CJ, Edwards DG (1986) Salinity effects on sunflower growth in solution culture. In: Proceedings of Australian Sunflower Association Workshop, 6th Gunnedah, New South Wales. Australian Sunflower Association, Toowoomba, Australia, pp 67–71
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307e–319e
Varshney RK, Bansal KC, Aggarwal PK, Datta SK, Craufurd PQ (2011) Agricultural biotechnology for crop improvement in a variable climate: hope or hype? Trends Plant Sci 16:363–371
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Doyle J, Doyle J (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Naz R, Bano A (2015) Molecular and physiological responses of sunflower (Helianthus annuus L.) to PGPR and SA under salt stress. Pak J Bot 47:35–42
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoloxidase in Beta vulgaris L. Plant Physiol 24:1–15
Bates LS, Waldern R, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Ebrahimian E, Bybordi A (2011) Exogenous silicium and zinc increase antioxidant enzyme activity and alleviate salt stress in leaves of sunflower. J Food Agric Environ 9:422–427
Cabello P, Agüera E, De La Ha P (2006) Metabolic changes during natural ageing in sunflower (Helianthus annuus) leaves: expression and activity of glutamine synthetase isoforms are regulated differently during senescence. Physiol Plant 128:175–185
Roumiana VI, Lydia S, Boris K, Tanya K (2014) Response of sunflower (Helianthus annuus L.) genotypes to PEG-mediated water stress. Cent Eur J Biol 9:1206–1214
Shahbaz M, Ashraf M, Aisha AN, Hanif A, Hameed S, Joham S, Rehman R (2011) Salt-induced modulation in growth, photosynthetic capacity, proline content and ion accumulation in sunflower (Helianthus annuus L.). Acta Physiol Plant 33:1113
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol 51:463–499
Dupont F (1992) Salt-induced changes in ion transport: regulation of primary pumps and secondary transporters. In: Cooke D, Clarkson D (eds) Transport and receptor proteins of plant membranes. Plenum Press, New York, pp 91–100
Hurkman WJ (1992) Effect of salt stress on germination expression: a review. Plant Soil 146:145–151
Dubey RS (2005) Photosynthesis in plants under stress full conditions. In: Pessarakli M (ed) Photosynthesis. CRC Press, NewYork, pp 717–718
Akram NA, Ashraf M (2011) Improvement in growth, chlorophyll pigments and photosynthetic performance in salt-stressed plants of sunflower (Helianthus annuus L.) by foliar application of 5-aminolevulinic acid. Agrochimica 55:94–104
Ashraf M, Sultana R (2000) Combination effect of NaCl salinity and N-form on mineral composition of sunflower plants. Biol Plant 43:615–619
Wenxue W, Bilsborrow PE, Hooley P, Fincham DA, Lombi E, Forster BP (2003) Salinity induced differences in growth, ion distribution and partitioning in barley between the cultivar Maythorpe and its derived mutant Golden Promise. Plant Soil 250:183–191
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Garcia AB, de Almeida EJ, Iyer S, Gerats T, Van MM, Caplan AB (1997) Effects of osmoprotectants upon NaCl in rice. Plant Physiol 115:159–169
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stress. Plant Cell 7:1109–1111
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ 35:259–270
Kumar S, Kalita A, Srivastava R, Sahoo L (2017) Co-expression of Arabidopsis NHX1 and bar improves the tolerance to salinity, oxidative stress, and herbicide in transgenic mungbean. Front Plant Sci 8:1896
Yazici I, Türkan I, Sekmen AH, Demiral T (2007) Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ Exp Bot 61:49–57
Acknowledgements
The authors acknowledge the Head, Department of Plant Sciences, University of Hyderabad for access to the research facilities provided by DST-FIST, DBT-CREBB, and UGC-SAP in undertaking this investigation. Authors also thank Prof.Souyi Chen and Jinsong Zhang, Institute of Genetics and Developmental Biology, CAS, Beijing for providing the pBin438-TaNHX2 construct. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
RY and PBK conceived the experiments. RM and RY performed the experiments. RM, RY, and PBK analyzed the data. RY and PBK wrote the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mushke, R., Yarra, R. & Kirti, P.B. Improved salinity tolerance and growth performance in transgenic sunflower plants via ectopic expression of a wheat antiporter gene (TaNHX2). Mol Biol Rep 46, 5941–5953 (2019). https://doi.org/10.1007/s11033-019-05028-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05028-7