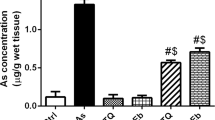
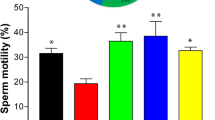
Abstract
Arsenic is well known genotoxicant which causes the excessive generation of reactive oxygen species (ROS) and inhibition of antioxidant enzyme systems leading to cell damage through the activation of oxidative sensitive signaling pathways. Epigallocatechin gallate (EGCG), the main and active polyphenolic catechin present in green tea, has shown potent antioxidant, free radical scavenging and genoprotective activity in vivo. The present study attempted to investigate antioxidant and geno-protective efficacy of EGCG by regulating arsenic induced oxidative stress in mice. Animals received prophylactic and therapeutic treatments at two different doses (25 and 50 mg/kg b.wt.) of EGCG orally for 15 days and administered arsenic intraperitoneally at dose of 1.5 mg/kg b.wt (1/10th of LD50) for 10 days. Arsenic intoxication revealed enhanced ROS production (114%) in lymphocytes; elevated levels of LPO (2–4 fold); reduced levels of hepato-renal antioxidants (approx. 45%) and augmented genomic fragmentation in hepato-renal tissues; increased chromosomal anomalies (78%) and micronucleation (21.93%) in bone marrow cells and comet tailing (25%) in lymphocytes of mice. Both pre and post treatments of EGCG decreased ROS production, restored lipid peroxidation (LPO) and reduced hepato-renal antioxidants levels, reduced the DNA fragmentation, number of chromosomal aberrations (CA), micronucleation (MN), and comet tailing but prophylactic treatment of 50 mg/kg b.wt was the most effective treatment in regulating arsenic induced oxidative stress. The effectiveness of this dose was furthermore validated by calculating the inhibitory index. Thus, results of present work empirically demonstrate free radical scavenging, anti-oxidative and genoprotective efficacy of EGCG against arsenic toxicity.







Similar content being viewed by others
References
Roy JS, Chatterjee D, Das N, Giri AK (2018) Substantial evidence indicate that arsenic is a genotoxic carcinogen: a review. Toxicol Res 34:311–324
Parkash C, Kumar V (2016) Chronic arsenic exposure-induced oxidative stress is mediated by decreased mitochondrial biogenesis in rat liver. Biol Trace Elem Res 173:87–95
Brinkel J, Khan MH, Kraemer A (2009) A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health 6:1609–1619
Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Cohen SM, Arnold LL, Beck BD, Lewis AS, Eldan M (2013) Evaluation of the carcinogenicity of inorganic arsenic. Crit Rev Toxicol 43:711–752
Mahata J, Basu A, Ghoshal S, Sarkar JN, Roy AK, Poddar G, Nandy AK, Banerjee A, Ray K, Natarajan AT, Nilsson R, Giri AK (2003) Chromosomal aberrations and sister chromatid exchanges in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat Res 534:133–143
Hei TK, Liu SX, Waldren C (1998) Mutagenicity of arsenic in mammalian cells: role of reactive oxygen species. Proc Natl Acad Sci USA 95:8103–8107
Faita F, Cori L, Bianchi F, Andreassi MG (2013) Arsenic-induced genotoxicity and genetic susceptibility to arsenic-related pathologies. Int J Environ Res Public Health 10:1527–1546
Mo J, Xia Y, Wade TJ, Schmitt M, Le XC, Dang R, Mumford JL (2006) Chronic arsenic exposure and oxidative stress: OGG1 expression and arsenic exposure, nail selenium and skin hyperkeratosis in Inner Mongolia. Environ Health Perspect 114:835–841
Hossain MB, Vahter M, Concha G, Broberg K (2012) Environmental arsenic exposure and DNA methylation of the tumor suppressor gene p16 and the DNA repair gene MLH1: effect of arsenic metabolism and genotype. Metallomics 4:1167–1175
Sun HJ, Rathinasabapathi B, Wu B, Luo J, Pu LP, Ma LQ (2014) Arsenic and selenium toxicity and their interactive effects in humans. Environ Int 69:148–158
Johnson JJ, Bailey HH, Mukhtar H (2010) Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine 17:3–13
Park SY, Jeong YJ, Kim SH, Jung JY, Kim WJ (2013) Epigallocatechin gallate protects against nitric oxide-induced apoptosis via scavenging ROS and modulating the Bcl-2 family in human dental pulp cells. J Toxicol Sci 38:371–378
Garcia-Rodriguez Mdel C, Montano-Rodriguez AR, Altamirano-Lozano MA (2016) Modulation of hexavalent chromium-induced genotoxic damage in peripheral blood of mice by epigallocatechin-3-gallate (EGCG) and its relationship to the apoptotic activity. J Toxicol Environ Health 79:28–38
Yang WS, Moon SY, Lee MJ, Park SK (2016) Epigallocatechin-3-gallate attenuates the effects of TNF-α in vascular endothelial cells by causing ectodomain shedding of TNF receptor 1. Cell Physiol Biochem 38:963–974
Chen J, Du L, Li J, Song H (2016) Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem Toxicol 96:70–78
Cabrera C, Artacho R, Gimenez R (2006) Beneficial effects of green tea-a review. J Am Col Nutr 25:79–99
OECD (2001) OECD guidelines for testing of chemicals 423
Kaushal S, Garg V, Ahsan AU, Sharma VL, Chopra M (2017) Alleviation of arsenic induced lung toxicity by Ocimum sanctum in murine model. Int J Pharm Sci Res 8:4604–4613
Orsolic N, Sirovina D, Gajski G, Garaj-Vrhovac V, Jazvinscak Jembrek M, Kosalec I (2013) Assessment of DNA damage and lipid peroxidation in diabetic mice: effects of propolis and epigallocatechin gallate (EGCG). Mutat Res 757:36–44
Bartosikova L, Necas J (2018) Epigallocatechin gallate: a review. Vet Med 63:443–467
Turner PV, Brabb T, Pekow C, Vasbinder MA (2011) Administration of substance to animal laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613
Beuge JA, Augst SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Beulter E, Duron O, Kelly BM (1963) Improved method for determination of blood glutathione. J Lab Clin Med 61:882–888
Luck H (1971) Catalase. Methods of enzymatic analysis. Academic Press, New York, pp 885–893
Kono Y (1978) Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophy 86:189–195
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Horn HD (1971) Glutathione reductase. Methods of enzymatic analysis. Academic Press, New York, pp 875–881
Sambrook J, Fritsh EF, Maniatis T (1989) A laboratory manual. Molecular cloning, 2nd edn. Cold Spring Harbour Laboratory Press, New York
Das KC (1966) Study of human chromosomes by a direct method from bone marrow. Ind J Pediatr 33:264–266
Hayashi M, Sofuni JI, Ishidate M (1983) An application of acridine orange fluorescent staining to the micronucleus test. Mutat Res 120:241–247
Singh NP, McCoy MT, Tice RR, Schneider EL (1989) A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Madrigal-Bujaidar E, Barriga SD, Cassani M, Marquez P, Revuelta P (1998) In vivo and in vitro antigenotoxic effect of nordihydroguaiaretic acid against SCEs induced by methyl methanesulfonate. Mutat Res 419:163–168
Kitchin KT, Conolly R (2010) Arsenic-induced carcinogenesis-oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem Res Toxicol 23:327–335
Pierce BL, Kibriya MG, Jasmine F, Argos M, Roy S, Paul-Brutus R, Rahaman R, Rakibuz-Zaman M, Parvez F, Ahmed A, Quasem I, Hore SK, Alam S, Islam T, Slavkovich V, Gamble MV, Yunus M, Rahman M, Baron JA, Graziano JH, Ahsan H (2012) Genome-wide association study identifies chromosome 10q24 32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet 8:1002522
Maheshwari N, Khan FH, Mahmood R (2018) 3,4-Dihydroxybenzaldehyde lowers ROS generation and protects human red blood cells from arsenic (III) induced oxidative damage. Environ Toxicol 33:861–875
Dutta S, Saha S, Mahalanoish S, Sadhukhan P, Sil PC (2018) Melatonin attenuates arsenic induced nephropathy via regulation of oxidative stress and inflammatory signalling cascade in mice. Food Chem Toxicol 118:303–316
Kenyon EM, Hughes MF, Adair BM, Highfill JH, Crecelius EA, Clewell HJ, Yager JW (2008) Tissue distribution and urinary excretion of inorganic arsenic and its methylated metabolites in C57BL6 mice following subchronic exposure to arsenate in drinking water. Toxicol Appl Pharmacol 232:448–455
Dkhil MA, Al-Khalifa MS, Al-Quraishy S, Zrieq R, Abdel Moneim AE (2016) Indigofera oblongifolia mitigates lead-acetate induced kidney damage and apoptosis in a rat model. Drug Des Dev Ther 10:1847–1856
Kharroubi W, Dhibi M, Mekni M, Haouas Z, Chreif I, Neffati F, Hammami M, Sakly R (2014) Sodium arsenate induced changes in fatty acids profiles and oxidative damage in kidney of rats. Environ Sci Pollut Res Int 21:12040–12049
Dua TK, Dewanjee S, Feo VD (2015) Ameliorative effect of water spinach, Ipomea aquatica (Convolvulaceae), against experimentally induced arsenic toxicity. J Transl Med 13:81–98
Laborde E (2010) Glutathione transferases as mediators of signaling pathways involvedin cell proliferation and cell death. Cell Death Differ 17:1373–1380
Muthumani M, Miltonprabu S (2015) Ameliorative efficacy of tetrahydrocurcumin against arsenic induced oxidative damage, dyslipidemia and hepatic mitochondrial toxicity in rats. Chem Biol Interact 235:95–105
Al-Brakati AY, Kassab RB, Lokman MS, Elmahallawy EK, Amin HK, Abdel Moneim AE (2018) Role of thymoquinone and ebselen in the prevention of sodium arsenite induced nephrotoxicity in female rats. Hum Exp Toxicol 38:482–493
Mehrzadi S, Fatemi I, Malayeri AR, Khodadadi A, Mohammadi F, Mansouri E, Rashno M, Goudarzi M (2018) Ellagic acid mitigates sodium arsenite renal and hepatic toxicity in male Wistar rats. Pharm Rep 70:712–719
Salazar AM, Miller HL, McNeely SC, Sordo M, Ostrosky-Wegman P, States JC (2010) Suppression of p53 and p21 reduces arsenite-induced aneuploidy. Chem Res Toxicol 23:357–364
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation and disease. FASEB J 17:1195–1214
Chopra M, Sobti RC, Sharma VL (2013) Cadmium administration mediated renal toxicity and plausible correlative chromosomal aberrations in mice. Int J Pharmacol Toxicol Sci 3:19–31
Sankar P, Telang AG, Ramya K, Vijayakaran K, Kesavan M, Sarkar SN (2014) Protective action of curcumin and nano-curcumin against arsenic induced genotoxicity in rats in vivo. Mol Biol Rep 41:7413–7422
Odunola OA, Akinwumi KA, Ibegbu DM (2011) The influence of garlic spondias mombin on sodium arsenite induced clastogenicity hepatotoxicity in rats. Pacific J Sci Technol 12:401–409
Tian D, Ma H, Feng Z, Xia Y, Le XC, Ni Z, Allen J, Collins B, Schreinemachers D, Mumford JL (2001) Analyses of micronuclei in exfoliated epithelial cells from individuals chronically exposed to arsenic via drinking water in Inner Mongolia, China. J Toxicol Environ Health 64:473–484
Titenko-Holland N, Moore LE (1994) Measurement and characterization of micronuclei in exfoliated human cells by fluorescence in situ hybridization with a centromeric probe. Mutat Res 312:39–59
Basu A, Mahata J, Roy AK, Sarkar JN, Poddar G, Nandy AK, Sarkar PK, Dutta PK, Banerjee A, Das M, Ray K, Roychaudhury S, Natarajan AT, Nilsson R, Giri AK (2002) Enhanced frequency of micronuclei in individuals exposed to arsenic through drinking water in West Bengal, India. Mutat Res 516:29–40
Ochi T, Suzuki T, Barrett JC, Tsutsui T (2004) A trivalent dimethylarsenic compound, dimethylarsine oxide, induces cellular transformation, aneuploidy, centrosome abnormality and multipolar spindle formation in Syrian hamster embryo cells. Toxicology 203:n155–n163
Gebel TW (2001) Genotoxicity of arsenical compounds. Int J Hyg Environ Health 203:249–262
Balakumar BS, Ramanathan K, Kumaresan S, Suresh R (2010) DNA damage by sodium arsenite in experimental rats: ameliorative effects of antioxidant vitamins C, E. Indian J Sci Technol 3:322–327
Sun T, Liu Z, Qi Z, Huang YP, Gao XQ, Zhang YY (2016) Epigallocatechin-3-gallate (EGCG) attenuates arsenic-induced cardiotoxicity in rats. Food Chem Toxicol 93:102e110
Miltonprabu S, Thangapandiya S (2015) Epigallocatechin gallate potentially attenuates fluoride induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. J Trace Elem Med Biol 29:321–335
Zhong Y, Shahidi F (2011) Lipophilized epigallocatechin gallate (EGCG) derivatives as a novel antioxidants. J Agric Food Chem 59:6526–6533
Thangapandiyan S, Miltonprabu S (2013) Epigallocatechin gallate effectively ameliorates fluoride-induced oxidative stress and DNA damage in the liver of rats. Can J Physiol Pharmacol 91:528–537
Othman AI, Elkomy MM, El-Missiry MA, Dardor M (2017) Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction. Eur J Pharmacol 794:27–36
Johnson MK, Loo G (2000) Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat Res 459:211–218
Abib RT, Peres KC, Barbosa AM, Peres TV, Bernardes A, Zimmermann LM, Quincozes-Santos A, Fiedler HD, Leal RB, Farina M, Gottfried C (2011) Epigallocatechin-3-gallate protects rat brain mitochondria against cadmium-induced damage. Food Chem Toxicol 49:2618–2623
Thangapandiyan S, Miltonprabu S (2014) Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: Role of Nrf2/Ho-1 signaling. Toxicol Rep 1:12–30
Devika PT, Prince SM (2008) (-) Epigalllocatechin-gallate (EGCG) prevents mitochondrial damage in isoproterenol-induced cardiac toxicity in albino Wistar rats: a transmission electron microscopic and in vitro study. Pharm Res 57:351–357
Acknowledgements
The authors are thankful to University Grant Commission-Rajiv Gandhi National Fellowship (F1-17.1/2012-13-RGNF-2012-13-SC-HIM-22387, dated 28-02-2013), University Grant Commission-Centre for Advanced Studies (F.4-28/2015/CAS-II (SAP-II, dated 20-07-2013) and Department of Science and Technology-PURSE for providing financial support to conduct this experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaushal, S., Ahsan, A.U., Sharma, V.L. et al. Epigallocatechin gallate attenuates arsenic induced genotoxicity via regulation of oxidative stress in balb/C mice. Mol Biol Rep 46, 5355–5369 (2019). https://doi.org/10.1007/s11033-019-04991-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04991-5