Abstract
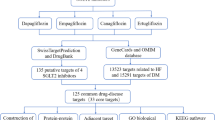
Acute kidney injury (AKI) is a global health concern with high incidence and mortality, where diabetes further worsens the condition. The available treatment options are not uniformly effective against the complex pathogenesis of AKI–diabetes comorbidity. Hence, combination therapies based on the multicomponent, multitarget approach can tackle more than one pathomechanism and can aid in AKI–diabetes comorbidity management. This study aimed to investigate the therapeutic potential of esculetin and phloretin combination against AKI–diabetes comorbidity by network pharmacology followed by validation by molecular docking and dynamics. The curative targets for diabetes, AKI, esculetin, and phloretin were obtained from DisGeNET, GeneCards, SwissTargetPrediction database. Further, the protein–protein interaction of the potential targets of esculetin and phloretin against AKI–diabetes comorbidity was investigated using the STRING database. Gene ontology and pathway enrichment analysis were performed with the help of the DAVID and KEGG databases, followed by network construction and analysis via Cytoscape. Molecular docking and dynamic simulations were performed to validate the targets of esculetin and phloretin against AKI–diabetes comorbidity. We obtained 6341 targets for AKI–diabetes comorbidity. Further, a total of 54 and 44 targets of esculetin and phloretin against AKI–diabetes comorbidity were retrieved. The top 10 targets for esculetin selected based on the degree value were AKR1B1, DAO, ESR1, PLK1, CA3, CA2, CCNE1, PRKN, HDAC2, and MAOA. Similarly, phloretin’s 10 key targets were ACHE, CDK1, MAPK14, APP, CDK5R1, CCNE1, MAOA, MAOB, HDAC6, and PRKN. These targets were enriched in 58 pathways involved in the pathophysiology of AKI–diabetes comorbidity. Further, esculetin and phloretin showed an excellent binding affinity for these critical targets. The findings of this study suggest that esculetin and phloretin combination as a multicomponent multitarget therapy has the potential to prevent AKI–diabetes comorbidity.
Graphical abstract









Similar content being viewed by others
Abbreviations
- AKI :
-
Acute kidney injury
- ACHE :
-
Acetylcholinesterase
- AKR1B1 :
-
Aldo–keto reductase family 1, member B1
- APP :
-
Amyloid precursor protein
- ARG :
-
Arginine
- ASN :
-
Asparagine
- ASP :
-
Aspartic acid
- CA3 :
-
Carbonic anhydrase 3
- CA2 :
-
Carbonic anhydrase 2
- CCNE1 :
-
Cyclin E1
- CDK1 :
-
Cyclin-dependent kinase 1
- CDK5R1 :
-
Cyclin-dependent kinase 5 regulatory subunit 1
- CYS :
-
Cysteine
- DAO :
-
D-amino acid oxidase
- ESR1 :
-
Oestrogen receptor 1
- GLN :
-
Glutamine
- GLU :
-
Glutamic acid
- HDAC6 :
-
Histone deacetylase 6
- HIE :
-
Histidine
- ILE :
-
Isoleucine
- LEU :
-
Leucine
- LYS :
-
Lysine
- MAOA :
-
Monoamine oxidase A
- MAOB :
-
Monoamine oxidase B
- MAPK14 :
-
Mitogen-activated protein kinase 14
- PHE :
-
Phenylalanine
- PLK1 :
-
Polo-like kinase 1
- PRKN :
-
Parkin RBR E3 Ubiquitin Protein Ligase
- PRO :
-
Proline
- SER :
-
Serine
- THR :
-
Threonine
- TRP :
-
Tryptophan
- TYR :
-
Tyrosine
References
Kellum JA et al (2021) Acute kidney injury. Nat Rev Dis Primers 7(1):1–17
Tai CW et al (2022) Acute kidney injury: epidemiology and course in critically ill children. J Nephrol 35(2):559–565
Lee S et al (2021) Intraoperative hyperglycemia in patients with an elevated preoperative C-reactive protein level may increase the risk of acute kidney injury after cardiac surgery. J Anesth 35(1):10–19
Gui Y et al (2023) Acute kidney injury in diabetes mellitus: epidemiology, diagnostic, and therapeutic concepts. FASEB J 37(4):e22884
Kaur A, Sharma GS, Kumbala DR (2023) Acute kidney injury in diabetic patients: a narrative review. Medicine 102(21):e33888
Harding JL et al (2020) US trends in hospitalizations for dialysis-requiring acute kidney injury in people with versus without diabetes. Am J Kidney Dis 75(6):897–907
Donderski R, Bednarski R, Manitius J (2020) Controversy over renin–angiotensin–aldosterone system (RAAS) inhibitors treatment in nephrology and cardiovascular diseases. Arter Hypertens 24(2):45–55
Dagar N, Kale A, Steiger S, Anders HJ, Gaikwad, AB (2022) Receptor-mediated mitophagy: an emerging therapeutic target in acute kidney injury. Mitochondrion 66:82–91
Zhang R et al (2019) Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol 10:123
Xin W et al (2021) TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med 19(1):1–11
Dagar N et al (2023) Nutraceuticals and network pharmacology approach for acute kidney injury: a review from the drug discovery aspect. Fitoterapia 168:105563
Li Q et al (2021) Effect of berberine on hyperuricemia and kidney injury: a network pharmacology analysis and experimental validation in a mouse model. Drug Des Dev Ther 15:3241
Oh KK, Adnan M, Cho DH (2021) Network pharmacology study on Morus alba L. leaves: pivotal functions of bioactives on RAS signaling pathway and its associated target proteins against Gout. Int J Mol Sci 22(17):9372
Memije-Lazaro IN et al (2018) Arthrospira maxima (Spirulina) and C-phycocyanin prevent the progression of chronic kidney disease and its cardiovascular complications. J Funct Foods 43:37–43
Zhang H et al (2021) Mechanistic insights into the renoprotective role of curcumin in cisplatin-induced acute kidney injury: network pharmacology analysis and experimental validation. Bioengineered 12(2):11039–11054
Mtewa AG et al (2021) Phytopharmaceuticals: efficacy, safety, and regulation. In: Preparation of phytopharmaceuticals for the management of disorders. Elsevier, pp 25–38
Chaturvedi S et al (2022) Applications of phytopharmaceuticals in targeting metabolic disorders. In: Drug delivery systems for metabolic disorders. Elsevier, pp 425–432
Balaha M, Kandeel S, Kabel A (2018) Phloretin either alone or in combination with duloxetine alleviates the STZ-induced diabetic neuropathy in rats. Biomed Pharmacother 101:821–832
Ying Y et al (2018) Phloretin prevents diabetic cardiomyopathy by dissociating Keap1/Nrf2 complex and inhibiting oxidative stress. Front Endocrinol 9:774
Kadakol A et al (2015) Esculetin reverses histone H2A/H2B ubiquitination, H3 dimethylation, acetylation and phosphorylation in preventing type 2 diabetic cardiomyopathy. J Funct Foods 17:127–136
Kadakol A et al (2017) Esculetin ameliorates insulin resistance and type 2 diabetic nephropathy through reversal of histone H3 acetylation and H2A lysine 119 monoubiquitination. J Funct Foods 35:256–266
Jung WK et al (2022) Antioxidant efficacy of esculetin against tert-butyl hydroperoxide-induced oxidative stress in HEK293 cells. Curr Issues Mol Biol 44(12):5986–5994
Güvenç M et al (2024) Protective effects of esculetin against ovary ischemia–reperfusion injury model in rats. J Biochem Mol Toxicol 38(1):e23528
Danis A et al (2023) Esculetin alleviates pentylenetetrazole-induced seizures, cognitive impairment and pro-inflammatory cytokines and suppresses penicillin-induced epileptiform activity in rats. Life Sci 313:121300
Zhou G et al (2023) Esculetin improves murine mastitis induced by Streptococcus isolated from bovine mammary glands by inhibiting NF-κB and MAPK signaling pathways. Microb Pathog 185:106393
Xia M et al (2023) The coumarin-derivative esculetin protects against lipotoxicity in primary rat hepatocytes via attenuating jnk-mediated oxidative stress and attenuates free fatty acid-induced lipid accumulation. Antioxidants 12(11):1922
Shen X et al (2023) Esculetin alleviates inflammation, oxidative stress and apoptosis in intestinal ischemia/reperfusion injury via targeting SIRT3/AMPK/mTOR signaling and regulating autophagy. J Inflamm Res 16:3655–3667
Li B et al (2023) Phloretin ameliorates heart function after myocardial infarction via NLRP3/Caspase-1/IL-1β signaling. Biomed Pharmacother 165:115083
Kapoor S, Padwad YS (2023) Phloretin suppresses intestinal inflammation and maintained epithelial tight junction integrity by modulating cytokines secretion in in vitro model of gut inflammation. Cell Immunol 391:104754
Li X et al (2023) Phloretin alleviates doxorubicin-induced cardiotoxicity through regulating Hif3a transcription via targeting transcription factor Fos. Phytomedicine 120:155046
Hytti M et al (2023) Phloretin inhibits glucose transport and reduces inflammation in human retinal pigment epithelial cells. Mol Cell Biochem 478(1):215–227
Shen X et al (2017) Phloretin exerts hypoglycemic effect in streptozotocin-induced diabetic rats and improves insulin resistance in vitro. Drug Des Dev Ther 11:313
Shelke V, Kale A, Kulkarni YA, Gaikwad AB (2024) Phloretin: a comprehensive review of its potential against diabetes and associated complications. J Pharm Pharmacol 76(3):201–212
Daina A, Michielin O, Zoete V (2019) SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res 47(W1):W357–W364
Liu T et al (2007) BindingDB: a web-accessible database of experimentally determined protein–ligand binding affinities. Nucleic Acids Res 35(suppl_1):D198–D201
Piñero J et al (2016) DisGeNET: a comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res gkw943
Stelzer G et al (2016) The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinform 54(1):1–30
Szklarczyk D et al (2021) The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res 49(D1):D605–D612
Sherman BT et al (2022) DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res 50(W1):W216–W221
Imran M et al (2022) Molecular modeling guided drug designing for the therapeutic treatment of rheumatoid arthritis
Ala C, Joshi RP, Gupta P, Ramalingam S, Sankaranarayanan M (2024) Discovery of potent DNMT1 inhibitors against sickle cell disease using structural-based virtual screening, MM-GBSA and molecular dynamics simulation-based approaches. J Biomol Struct Dyn 42(1):261–273
Kikiowo B, Ahmad I, Alade AA, Ijatuyi T, Iwaloye O, Patel HM (2023) Molecular dynamics simulation and pharmacokinetics studies of ombuin and quercetin against human pancreatic α-amylase. J Biomol Struct Dyn 41(20):10388–10395
Dagar N et al (2022) Receptor-mediated mitophagy: an emerging therapeutic target in acute kidney injury. Mitochondrion 66:82–91
Shelke V et al (2022) Epigenetic regulation of Toll-like receptors 2 and 4 in kidney disease. J Mol Med 100(7):1017–1026
Shelke V et al (2023) Toll-like receptors 2 and 4 stress signaling and sodium-glucose cotransporter-2 in kidney disease. Mol Cell Biochem 478(9):1987–1998
Wen L et al (2023) Tubular aryl hydratocarbon receptor upregulates EZH2 to promote cellular senescence in cisplatin-induced acute kidney injury. Cell Death Dis 14(1):18
Li J et al (2023) Nephroprotective mechanisms of Rhizoma Chuanxiong and Radix et Rhizoma Rhei against acute renal injury and renal fibrosis based on network pharmacology and experimental validation. Front Pharmacol 14:1154743
Salama AA, Elgohary R, Fahmy MI (2023) Protocatechuic acid ameliorates lipopolysaccharide‐induced kidney damage in mice via downregulation of TLR‐4‐mediated IKBKB/NF‐κB and MAPK/Erk signaling pathways. J Appl Toxicol 43(8): 1119–1129
González-Soria I et al (2023) SerpinA3K deficiency reduces oxidative stress in acute kidney injury. Int J Mol Sci 24(9):7815
Cao Z et al (2023) Simultaneous blockade of VEGF-B and IL-17A ameliorated diabetic kidney disease by reducing ectopic lipid deposition and alleviating inflammation response. Cell Death Discovery 9(1):8
Liu C, Wang Q, Niu L (2023) Sufentanil inhibits Pin1 to attenuate renal tubular epithelial cell ischemia–reperfusion injury by activating the PI3K/AKT/FOXO1 pathway. Int Urol Nephrol 55(8):1903–1916
Zuo Z, Li Q, Zhou S, Yu R, Wu C, Chen J, Wang W (2023) Berberine ameliorates contrast‐induced acute kidney injury by regulating HDAC4‐FoxO3a axis‐induced autophagy: in vivo and in vitro. Phytother Res 18:7745–7758
Dare A, Channa ML, Nadar A (2021) L-ergothioneine and its combination with metformin attenuates renal dysfunction in type-2 diabetic rat model by activating Nrf2 antioxidant pathway. Biomed Pharmacother 141:111921
Shelke V et al (2023) Concomitant inhibition of TLR-4 and SGLT2 by phloretin and empagliflozin prevents diabetes-associated ischemic acute kidney injury. Food Funct 14(11):5391–5403
Wang Y et al (2021) Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res 28:231–243
Liu Z, Guan C, Li C, Zhang N, Yang C, Xu L, Xu Y (2022) Tilianin reduces apoptosis via the ERK/EGR1/BCL2L1 pathway in ischemia/reperfusion-Induced acute kidney injury mice. Front Pharmacol 13:862584
Pandey A et al (2017) Esculetin ameliorates hepatic fibrosis in high fat diet induced non-alcoholic fatty liver disease by regulation of FoxO1 mediated pathway. Pharmacol Rep 69(4):666–672
Liu J, Sun M, Xia Y, Cui X, Jiang J (2022) Phloretin ameliorates diabetic nephropathy by inhibiting nephrin and podocin reduction through a non-hypoglycemic effect. Food Funct 13(12):6613–6622
Luo T-T et al (2020) Network pharmacology in research of Chinese medicine formula: methodology, application and prospective. Chin J Integr Med 26(1):72–80
Lee S-K, Boron WF, Occhipinti R (2023) Potential novel role of membrane-associated carbonic anhydrases in the kidney. Int J Mol Sci 24(4):4251
Er A (2023) Various carbonic anhydrases in physiopathological events, carbonic anhydrase inhibitors, and hybrid compounds. Lett Drug Des Discovery 20(10):1427–1436
Liu L et al (2019) p53 upregulated by HIF-1 α promotes hypoxia-induced G2/M arrest and renal fibrosis in vitro and in vivo. J Mol Cell Biol 11(5):371–382
Wu T-T et al (2020) AKR1B1-induced epithelial–mesenchymal transition mediated by RAGE-oxidative stress in diabetic cataract lens. Antioxidants 9(4):273
Li L et al (2024) Proteomics-based screening of AKR1B1 as a therapeutic target and validation study for sepsis-associated acute kidney injury. PeerJ 12:e16709
Zhang H et al (2012) Study on the decrease of renal D-amino acid oxidase activity in the rat after renal ischemia by chiral ligand exchange capillary electrophoresis. Amino Acids 42:337–345
Ma H-Y, Chen S, Du Y (2021) Estrogen and estrogen receptors in kidney diseases. Ren Fail 43(1):619–642
Kim D-E et al (2023) Plk2-mediated phosphorylation and translocalization of Nrf2 activates anti-inflammation through p53/Plk2/p21cip1 signaling in acute kidney injury. Cell Biol Toxicol 39(4):1509–1529
Du Y et al (2023) Plk1 promotes renal tubulointerstitial fibrosis by targeting autophagy/lysosome axis. Cell Death Dis 14(8):571
Zhao F, Zhu J, Zhang M, Luo Y, Li Y, Shi L, Wu X (2023) OGG1 aggravates renal ischemia–reperfusion injury by repressing PINK1‐mediated mitophagy. Cell Prolif 56(8):e13418
Shi L et al (2022) HDAC6 inhibition alleviates ischemia-and cisplatin-induced acute kidney injury by promoting autophagy. Cells 11(24):3951
Meakin PJ et al (2020) Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction. J Clin Investig 130(8):4104–4117
Tanaka R et al (2017) Sex differences in ischaemia/reperfusion-induced acute kidney injury depends on the degradation of noradrenaline by monoamine oxidase. Clin Exp Pharmacol Physiol 44(3):371–377
Acknowledgements
ABG sincerely acknowledges the financial support provided by the Birla Institute of Technology and Science, Pilani, Pilani Campus, for carrying out this work.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
N.D. was involved in conceptualization, writing—original draft preparation, methodology, investigation, data analysis. H.R.J. helped in writing—review & editing of the manuscript. A.B.G. conceptualised and designed the experiments, writing—review & editing of the manuscript, supervision. All authors have approved the final manuscript draft for publication.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dagar, N., Jadhav, H.R. & Gaikwad, A.B. Network pharmacology combined with molecular docking and dynamics to assess the synergism of esculetin and phloretin against acute kidney injury-diabetes comorbidity. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10829-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10829-5