Abstract
In this study, a total of 18 new benzamide/ nicotinamide/ cinnamamide derivative compounds were designed and synthesized for the first time (except B1 and B5) by conventional and microwave irradiation methods. The chemical structures of the synthesized compounds were characterized by 1H NMR, 13C NMR, and HRMS spectra. In vitro acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibition effects of the compounds were evaluated to find out new possible drug candidate molecule/s. According to the inhibition results, the IC50 values of the compounds synthesized were in the range of 10.66–83.03 nM towards AChE, while they were in the range of 32.74–66.68 nM towards BuChE. Tacrine was used as the reference drug and its IC50 values were 20.85 nM and 15.66 nM towards AChE and BuChE, respectively. The most active compounds B4 (IC50: 15.42 nM), N4 (IC50: 12.14 nM), and C4 (IC50: 10.67 nM) in each series towards AChE were docked at the binding site of AChE enzyme to explain the inhibitory activities of each series. On the other hand, the compounds B4, N4, and C4 showed satisfactory pharmacokinetic properties via the prediction of ADME profiles.
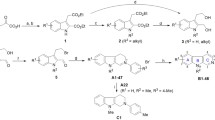
Graphic abstract





Similar content being viewed by others
References
Grabher BJ (2018) Effects of alzheimer disease on patients and their family. J Nucl Med Technol 46(4):335–340. https://doi.org/10.2967/jnmt.118.218057
Moodie Lindon WK, Sepčić K, Turk T, Frangež R, Svenson J (2019) Natural cholinesterase inhibitors from marine organisms. Nat Prod Rep 36(8):1053–1092. https://doi.org/10.1039/C9NP00010K
Liu P-P, Xie Y, Meng X-Y, Kang J-S (2019) History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct Target Ther 4(1):29. https://doi.org/10.1038/s41392-019-0063-8
Greig NH, Utsuki T, Yu QS, Zhu XX, Holloway HW, Perry T, Lee B, Ingram DK, Lahiri DK (2001) A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin 17(3):159–165
Moss DE, Perez RG, Kobayashi H (2017) Cholinesterase inhibitor therapy in alzheimer’s disease: the limits and tolerability of irreversible cns-selective acetylcholinesterase inhibition in primates. J Alzheimer’s Dis 55(3):1285–1294. https://doi.org/10.3233/Jad-160733
Bentley P, Driver J, Dolan RJ (2008) Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain 131:409–424. https://doi.org/10.1093/brain/awm299
Giacobini E, Spiegel R, Enz A, Veroff AE, Cutler NR (2002) Inhibition of acetyl- and butyryl-cholinesterase in the cerebrospinal fluid of patients with Alzheimer’s disease by rivastigmine: correlation with cognitive benefit. J Neural Transm 109(7–8):1053–1065. https://doi.org/10.1007/s007020200089
Mecocci P, Paolacci L, Boccardi V (2020) Chapter 29 - Cholinesterase inhibitors in dementias: an overview. In: Martin CR, Preedy VR (eds) Diagnosis and Management in Dementia. Academic Press, https://doi.org/10.1016/B978-0-12-815854-8.00029-X
Md. Tanvir K, Md. Sahab U, Mst. Marium B, Shanmugam T, Md. Sohanur R, Lotfi A, Bijo M, Muniruddin A, George EB, Ghulam Md A, (2019) Cholinesterase inhibitors for alzheimer’s disease: multitargeting strategy based on anti-alzheimer’s drugs repositioning. Curr Pharm Des 25(33):3519–3535. https://doi.org/10.2174/1381612825666191008103141
Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren Jan JGM (2019) Myasthenia gravis (Primer). Nat Rev Dis Primers. https://doi.org/10.1038/s41572-019-0079-y
Ragab HM, Teleb M, Haidar HR, Gouda N (2019) Chlorinated tacrine analogs: design, synthesis and biological evaluation of their anti-cholinesterase activity as potential treatment for Alzheimer’s disease. Bioorg Chem 86:557–568. https://doi.org/10.1016/j.bioorg.2019.02.033
Amine Khodja I, Boulebd H (2021) Synthesis, biological evaluation, theoretical investigations, docking study and ADME parameters of some 1,4-bisphenylhydrazone derivatives as potent antioxidant agents and acetylcholinesterase inhibitors. Mol Diversity 25(1):279–290. https://doi.org/10.1007/s11030-020-10064-8
Mehrazar M, Hassankalhori M, Toolabi M, Goli F, Moghimi S, Nadri H, Bukhari SNA, Firoozpour L, Foroumadi A (2020) Design and synthesis of benzodiazepine-1,2,3-triazole hybrid derivatives as selective butyrylcholinesterase inhibitors. Mol Diversity 24(4):997–1013. https://doi.org/10.1007/s11030-019-10008-x
Ghobadian R, Esfandyari R, Nadri H, Moradi A, Mahdavi M, Akbarzadeh T, Khaleghzadeh-Ahangar H, Edraki N, Sharifzadeh M, Amini M (2020) Design, synthesis, in vivo and in vitro studies of 1,2,3,4-tetrahydro-9H-carbazole derivatives, highly selective and potent butyrylcholinesterase inhibitors. Mol Diversity 24(1):211–223. https://doi.org/10.1007/s11030-019-09943-6
Straniero V, Suigo L, Casiraghi A, Sebastián-Pérez V, Hrast M, Zanotto C, Zdovc I, De Giuli Morghen C, Radaelli A, Valoti E (2020) Benzamide Derivatives Targeting the Cell Division Protein FtsZ: Modifications of the Linker and the Benzodioxane Scaffold and Their Effects on Antimicrobial Activity. Antibiotics 9 (4). Doi: https://doi.org/10.3390/antibiotics9040160
Perin N, Roškarić P, Sović I, Boček I, Starčević K, Hranjec M, Vianello R (2018) Amino-substituted benzamide derivatives as promising antioxidant agents: a combined experimental and computational study. Chem Res Toxicol 31(9):974–984. https://doi.org/10.1021/acs.chemrestox.8b00175
Makovec F, Peris W, Revel L, Giovanetti R, Redaelli D, Rovati LC (1992) Antiallergic and cytoprotective activity of new N-phenylbenzamido acid derivatives. J Med Chem 35(20):3633–3640. https://doi.org/10.1021/jm00098a006
Wajid S, Khatoon A, Khan MA, Zafar H, Kanwal S, Atta ur R, Choudhary MI, Basha FZ, (2019) Microwave-Assisted Organic Synthesis, structure–activity relationship, kinetics and molecular docking studies of non-cytotoxic benzamide derivatives as selective butyrylcholinesterase inhibitors. Bioorg Med Chem 27(18):4030–4040. https://doi.org/10.1016/j.bmc.2019.07.015
Gao X-h, Liu L-b, Liu H-r, Tang J-j, Kang L, Wu H, Cui P, Yan J (2018) Structure–activity relationship investigation of benzamide and picolinamide derivatives containing dimethylamine side chain as acetylcholinesterase inhibitors. J Enzyme Inhib Med Chem 33(1):110–114. https://doi.org/10.1080/14756366.2017.1399885
Rui M, Rossino G, Coniglio S, Monteleone S, Scuteri A, Malacrida A, Rossi D, Catenacci L, Sorrenti M, Paolillo M, Curti D, Venturini L, Schepmann D, Wünsch B, Liedl KR, Cavaletti G, Pace V, Urban E, Collina S (2018) Identification of dual Sigma1 receptor modulators/acetylcholinesterase inhibitors with antioxidant and neurotrophic properties, as neuroprotective agents. Eur J Med Chem 158:353–370. https://doi.org/10.1016/j.ejmech.2018.09.010
Çatak J (2019) Determination of niacin profiles in some animal and plant based foods by high performance liquid chromatography: association with healthy nutrition. J Anim Sci Technol 61(3):138–146. https://doi.org/10.5187/jast.2019.61.3.138
Belenky P, Bogan KL, Brenner C (2007) NAD+ metabolism in health and disease. Trends Biochem Sci 32(1):12–19. https://doi.org/10.1016/j.tibs.2006.11.006
Fricker RA, Green EL, Jenkins SI, Griffin SM (2018) The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int J Tryptophan Res 11:117864691877665. https://doi.org/10.1177/1178646918776658
Moraski GC, Oliver AG, Markley LD, Cho S, Franzblau SG, Miller MJ (2014) Scaffold-switching: An exploration of 5,6-fused bicyclic heteroaromatics systems to afford antituberculosis activity akin to the imidazo[1,2-a]pyridine-3-carboxylates. Bioorg Med Chem Lett 24(15):3493–3498. https://doi.org/10.1016/j.bmcl.2014.05.062
Wang A, Lv K, Li L, Liu H, Tao Z, Wang B, Liu M, Ma C, Ma X, Han B, Wang A, Lu Y (2019) Design, synthesis and biological activity of N-(2-phenoxy)ethyl imidazo[1,2-a]pyridine-3-carboxamides as new antitubercular agents. Eur J Med Chem 178:715–725. https://doi.org/10.1016/j.ejmech.2019.06.038
Ni T, Li R, Xie F, Zhao J, Huang X, An M, Zang C, Cai Z, Zhang D, Jiang Y (2017) Synthesis and biological evaluation of novel 2-aminonicotinamide derivatives as antifungal agents. ChemMedChem 12(4):319–326. https://doi.org/10.1002/cmdc.201600545
Zhang H, Lu X, Zhang L-R, Liu J-J, Yang X-H, Wang X-M, Zhu H-L (2012) Design, synthesis and biological evaluation of N-phenylsulfonylnicotinamide derivatives as novel antitumor inhibitors. Bioorg Med Chem Lett 20(4):1411–1416. https://doi.org/10.1016/j.bmc.2012.01.004
Majellaro M, Stefanachi A, Tardia P, Vicenti C, Boccarelli A, Pannunzio A, Campanella F, Coluccia M, Denora N, Leonetti F, De Candia M, Altomare CD, Cellamare S (2017) Investigating structural requirements for the antiproliferative activity of biphenyl nicotinamides. ChemMedChem 12(16):1380–1389. https://doi.org/10.1002/cmdc.201700365
Peng M, Shi L, Ke S (2017) Nicotinamide-based diamides derivatives as potential cytotoxic agents: synthesis and biological evaluation. Chem Cent J 11 (1). doi:https://doi.org/10.1186/s13065-017-0338-5
Kishore A, Nampurath GK, Mathew SP, Zachariah RT, Potu BK, Rao MS, Valiathan M, Chamallamudi MR (2009) Antidiabetic effect through islet cell protection in streptozotocin diabetes: A preliminary assessment of two thiazolidin-4-ones in Swiss albino mice. Chem-Biol Interact 177(3):242–246. https://doi.org/10.1016/j.cbi.2008.10.032
Yilmaz Z, Piracha F, Anderson L, Mazzola N (2017) Supplements for Diabetes Mellitus: A Review of the Literature. J Res Pharm Pract 30(6):631–638. https://doi.org/10.1177/0897190016663070
Ruf S, Hallur MS, Anchan NK, Swamy IN, Murugesan KR, Sarkar S, Narasimhulu LK, Putta VPRK, Shaik S, Chandrasekar DV, Mane VS, Kadnur SV, Suresh J, Bhamidipati RK, Singh M, Burri RR, Kristam R, Schreuder H, Czech J, Rudolph C, Marker A, Langer T, Mullangi R, Yura T, Gosu R, Kannt A, Dhakshinamoorthy S, Rajagopal S (2018) Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg Med Chem Lett 28(5):922–925. https://doi.org/10.1016/j.bmcl.2018.01.058
Kwak JY, Ham HJ, Kim CM, Hwang ES (2015) Nicotinamide exerts antioxidative effects on senescent cells. Mol Cells 38(3):229–235. https://doi.org/10.14348/molcells.2015.2253
Williams PA, Harder JM, John SWM (2017) Glaucoma as a metabolic optic neuropathy: making the case for nicotinamide treatment in glaucoma. J Glaucoma 26(12):1161–1168. https://doi.org/10.1097/IJG.0000000000000767
Chi Y, Sauve AA (2013) Nicotinamide riboside, a trace nutrient in foods, is a Vitamin B3 with effects on energy metabolism and neuroprotection. Current Opinion in Clinical Nutrition & Metabolic Care 16 (6)
Spector R (1987) Niacinamide transport through the blood-brain-barrier. Neurochem Res 12(1):27–31. https://doi.org/10.1007/Bf00971360
Xie X, Gao Y, Zeng M, Wang Y, Wei T-F, Lu Y-B, Zhang W-P (2019) Nicotinamide ribose ameliorates cognitive impairment of aged and Alzheimer’s disease model mice. Metab Brain Dis 34(1):353–366. https://doi.org/10.1007/s11011-018-0346-8
Lee HJ, Yang SJ (2019) Supplementation with nicotinamide riboside reduces brain inflammation and improves cognitive function in diabetic mice. Int J Mol Sci 20(17):4196. https://doi.org/10.3390/ijms20174196
Yao Z, Yang W, Gao Z, Jia P (2017) Nicotinamide mononucleotide inhibits JNK activation to reverse Alzheimer disease. Neurosci Lett 647:133–140. https://doi.org/10.1016/j.neulet.2017.03.027
Rennie G, Chen AC, Dhillon H, Vardy J, Damian DL (2015) Nicotinamide and neurocognitive function. Nutr Neurosci 18(5):193–200. https://doi.org/10.1179/1476830514y.0000000112
Żurek E, Szymański P, Mikiciuk-Olasik E (2013) Synthesis and biological activity of new donepezil-hydrazinonicotinamide hybrids. Drug Research 63(03):137–144. https://doi.org/10.1055/s-0033-1333735
Narasimhan B, Belsare D, Pharande D, Mourya V, Dhake A (2004) Esters, amides and substituted derivatives of cinnamic acid: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 39(10):827–834. https://doi.org/10.1016/j.ejmech.2004.06.013
Ribeiro D, Poença C, Varela C, Janela J, Tavares Da Silva EJ, Fernandes E, Roleira FMF (2019) New phenolic cinnamic acid derivatives as selective COX-2 inhibitors. design, synthesis, biological activity and structure-activity relationships. Bioorg Chem 91:103179–103189. https://doi.org/10.1016/j.bioorg.2019.103179
De P, Koumba Yoya G, Constant P, Bedos-Belval F, Duran H, Saffon N, Daffe M, Baltas M (2011) Design, synthesis, and biological evaluation of new cinnamic derivatives as antituberculosis agents. J Med Chem 54(5):1449–1461. https://doi.org/10.1021/jm101510d
Romagnoli R, Baraldi PG, Salvador MK, Chayah M, Camacho ME, Prencipe F, Hamel E, Consolaro F, Basso G, Viola G (2014) Design, synthesis and biological evaluation of arylcinnamide hybrid derivatives as novel anticancer agents. Eur J Med Chem 81:394–407. https://doi.org/10.1016/j.ejmech.2014.05.028
Ullah S, Park Y, Ikram M, Lee S, Park C, Kang D, Yang J, Akter J, Yoon S, Chun P, Moon HR (2018) Design, synthesis and anti-melanogenic effect of cinnamamide derivatives. Bioorg Med Chem 26(21):5672–5681. https://doi.org/10.1016/j.bmc.2018.10.014
Ruwizhi N, Aderibigbe BA (2020) Cinnamic acid derivatives and their biological efficacy. Int J Mol Sci 21(16):5712. https://doi.org/10.3390/ijms21165712
Gunia-Krzyżak A, Żesławska E, Słoczyńska K, Żelaszczyk D, Sowa A, Koczurkiewicz-Adamczyk P, Popiół J, Nitek W, Pękala E, Marona H (2020) S(+)-(2E)-N-(2-Hydroxypropyl)-3-Phenylprop-2-Enamide (KM-568): a novel cinnamamide derivative with anticonvulsant activity in animal models of seizures and epilepsy. Int J Mol Sci 21(12):4372. https://doi.org/10.3390/ijms21124372
Xiao Y, Yang X, Li B, Yuan H, Wan S, Xu Y, Qin Z (2011) Design, synthesis and antifungal/insecticidal evaluation of novel cinnamide derivatives. Molecules 16(11):8945–8957. https://doi.org/10.3390/molecules16118945
Gaikwad N, Nanduri S, Madhavi YV (2019) Cinnamamide: An insight into the pharmacological advances and structure–activity relationships. Eur J Med Chem 181:111561–111558. https://doi.org/10.1016/j.ejmech.2019.07.064
Mishra P, Kumar A, Panda G (2019) Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). Bioorg Med Chem 27(6):895–930. https://doi.org/10.1016/j.bmc.2019.01.025
Xu W, Wang XB, Wang ZM, Wu JJ, Li F, Wang J, Kong LY (2016) Synthesis and evaluation of donepezil-ferulic acid hybrids as multi-target-directed ligands against Alzheimer’s disease. Medchemcomm 7(5):990–998. https://doi.org/10.1039/c6md00053c
Koca M, Yerdelen KO, Anil B, Kasap Z (2015) Microwave-assisted synthesis, molecular docking, and cholinesterase inhibitory activities of new ethanediamide and 2-butenediamide analogues. Chem Pharm Bull 63(3):210–217. https://doi.org/10.1248/cpb.c14-00754
Yerdelen KO, Koca M, Kasap Z, Anil B (2015) Preparation, anticholinesterase activity, and docking study of new 2-butenediamide and oxalamide derivatives. J Enzyme Inhib Med Chem 30(4):671–678. https://doi.org/10.3109/14756366.2014.959947
Yerdelen KO, Tosun E (2015) Synthesis, docking and biological evaluation of oxamide and fumaramide analogs as potential AChE and BuChE inhibitors. Med Chem Res 24(2):588–602. https://doi.org/10.1007/s00044-014-1152-4
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colourimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791. https://doi.org/10.1002/jcc.21256
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7(1):42717. https://doi.org/10.1038/srep42717
Bilginer S, Gul HI, Erdal FS, Sakagami H, Gulcin I (2020) New halogenated chalcones with cytotoxic and carbonic anhydrase inhibitory properties: 6-(3-Halogenated phenyl-2-propen-1-oyl)-2(3H)-benzoxazolones. Arch Pharm (Weinheim) 353(6):e1900384. https://doi.org/10.1002/ardp.201900384
Bilginer S, Gul HI, Anil B, Demir Y, Gulcin I (2020) Synthesis and in silico studies of triazene-substituted sulfamerazine derivatives as acetylcholinesterase and carbonic anhydrases inhibitors. Archiv der Pharmazie Doi: https://doi.org/10.1002/ardp.202000243
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23(1):3–25. https://doi.org/10.1016/S0169-409X(96)00423-1
Acknowledgements
I would like to thank Prof. Dr. Hasan Seçen for supporting this study with laboratory facilities, knowledge, and experience.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koca, M., Bilginer, S. New benzamide derivatives and their nicotinamide/cinnamamide analogs as cholinesterase inhibitors. Mol Divers 26, 1201–1212 (2022). https://doi.org/10.1007/s11030-021-10249-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10249-9