Abstract
An efficient and practical synthetic procedure for libraries of diversified 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones using a multicomponent process is presented. A convenient synthetic procedure for obtaining functionalized 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones via ring-opening strategy has also been developed. This protocol was found to be compatible with a wide range of substituents and paves the way for the practical synthesis of title compounds with a broad range of substituents under mild condition. The products can be easily isolated by crystallization without the use of chromatography.
Graphic abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Synthesis of privileged classes of heterocyclic compounds has become one of the prime areas of research in the field of synthetic and medicinal chemistry, as most of the biologically active compounds are derived from heterocyclic structures [1, 2]. The diversification of heterocyclic compounds using diversity-oriented synthesis (DOS) has been proved to be an essential tool for rapid discovery of small biologically active molecules [3, 4]. Therefore, the development of a concise and efficient strategy leading to skeletal and stereochemical diversity has gained much attention in scientific communities involved in drug discovery and biomedical research [5, 6].
In turn, benzopyrans (chromones) are important privileged structural motifs and serve as useful templates for the design of compound libraries in the search for novel biologically important compounds [7,8,9,10,11,12,13]. To date, attention was mostly focused on the synthesis of 2(3)-substituted and 2,3-disubstituted chromones. In contrast, synthetic methodologies to access chromone-fused rings are scarce, especially those involving construction of chromone-fused heterocyclic compounds. Existing reviews summarize synthetic approaches to fused pyrazoles [14,15,16], chromone-pyrazole-fused compounds [17], azachromones, azachromanones [18]. A unique pyrano[2,3-c]pyrrole bicyclic skeleton, exhibiting antioxidant activity [19,20,21,22,23] and rarely detected in nature, was found in Pyranonigrins—secondary metabolites produced by Aspergillus niger. Pyranonigrin A is also a potent inhibitor of the Main protease (Mpro) of the novel SARS-CoV-2 virus [24]. Compounds such as chromeno[2,3-c]pyrroles were reported to behave as glucokinase activators [25] and as mimetics of glycosaminoglycans [26] (Fig. 1).
To our knowledge, the chromeno[2,3-c]pyrrole scaffold has been rarely mentioned in the literature. Our previous reports focused on the limited examples of the synthesis of chromeno[2,3-c]pyrrole derivatives via multistep condensation of methyl 4-(o-hydroxyphenyl)-2,4-dioxobutanoate [27,28,29] and methyl (E)-6-phenyl-2,4-dioxohex-5-enoate [30] with aromatic aldehydes and aliphatic amines or ethyl 3-bromomethyl-4-oxochromene-2-carboxylate with amines [31]. Additionally, recent papers reported the synthesis of spiro derivatives of chromeno[2,3-c]pyrrole-3,9-diones with N-substituted isatins used as the carbonyl component [32, 33]. The acid-catalyzed transformation of some furo [3,4-b]chromones into pyrrolo[3,4-b]chromones has also been reported [34,35,36,37,38]. The multicomponent reaction of 3-formylchromones with isocyanides and azodicarboxylates is an alternative route to the preparation of chromeno[2,3-c]pyrroles [39]. It should be noted that one-pot multicomponent reactions (MCRs) are some of the most efficient tools in modern synthetic organic chemistry for the preparation of highly functionalized organic compounds [40], including N-heterocycles [41, 42]. Moreover, they have all the features of a perfect synthetic method: high efficiency and step economy, quick and simple implementation, time and energy reductions, environmental friendliness, and suitability for target and diversity-oriented synthesis [43].
Aiming at accessing diverse privileged skeletons of chromeno[2,3-c]pyrrole, we performed rapid and efficient construction of target libraries of drug-like compounds—2-alkyl-1-aryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones—from relatively simple and commercial available reagents via one-pot multicomponent heterocyclization.
Results and discussion
As part of our interest in the development of combinatorial chemistry, we aimed to design a previously unreported one-pot multicomponent reaction. Initially, as a model reaction, we assessed the three-component reaction of commercially available 4-(2-hydroxyphenyl)-2,4-dioxobutanoate 1{1}, benzaldehyde 2{1} and benzylamine 3{16}, to survey key parameters for one-pot process (Scheme 1). We first tested the reaction of starting reagents (1{1}, 2{1} and 3{16}) in the presence of different solvents to optimize the reaction conditions (Table 1). Indeed, upon interaction of the starting reagents in a molar ratio of 1:1:1 in 10 mL of absolute MeOH at room temperature for 1.5 h, the reaction gave 2-benzyl-1-phenyl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{1–1-16}) as the only product with a yield of 24% (Entry 1, Table 1). When the reaction was repeated at 40 °C, the same product 4{1–1-16} was obtained in the higher yield of 36% (Entry 2, Table 1). The reaction was repeated in EtOH and in EtOH with addition of 1 mL of acetic acid at 40 °C, which resulted in 4{1–1-16} with yields of 26% and 74%, respectively (Entry 3 and 4, Table 1). The product 4{1–1-16} also formed in low yields when using i-PrOH (42% yield of the product), THF (17% yield of the product) and acetic acid (22% yield of the product) at 40 °C (Entry 5–7, Table 1). It is noteworthy that in acetic acid under reflux, 4{1–1-16} was obtained in trace amounts in a complex mixture of unidentified products (Entry 8, Table 1). Overall, in almost all cases the yields of the key product were not high. Thus, the best approach (primarily based on the yield of the key reaction product) was to carry out the reaction in absolute EtOH with the addition of acetic acid at 40 °C, which completed in 30 min (Entry 4, Table 1). We also noted that when the reaction was carried out under these conditions with molar ratios of the starting reagents of 1:1.2:1.2 or 1:1.5:1.5, the product 4{1–1-16} formed with moderate yields (up to 36%) together with unidentified mixtures of complex products.
Based on the results of reaction optimization, we propose the following mechanism for this one-pot three-component reaction (Scheme 2). The condensation of starting compounds occurs under basic conditions and gives an adduct A, which is then converted into 1-alkyl-5-aryl-4-(2-hydroxybenzoyl)-3-hydroxy-2,5-dihydro-1H-pyrrol-2-one B. Subsequent dehydration of B results in the product 4. The dehydration is more effective in acidic medium; addition of 1 mL of acetic acid was sufficient to increase the yield, while adding bigger amounts resulted in higher reaction temperature and resulted in noticeably smaller yields.
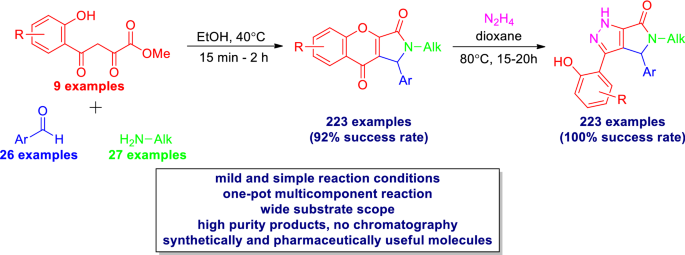
With the optimized reaction conditions in hand, we set out to explore the use of substituted methyl 4-(o-hydroxyphenyl)-2,4-dioxobutanoates 1{1–9} (9 examples), aryl aldehydes 2{1–26} (26 examples) and primary amines 3{1–27} (27 examples) in the obtainment of a variety of 2-alkyl-1-aryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones 4 in a one-pot multicomponent reaction (Scheme 3).
The representative substrates—methyl 4-(o-hydroxyphenyl)-2,4-dioxobutanoates 1{1–9}, aryl aldehydes 2{1–26} and primary amines 3{1–27}—are listed in Fig. 2. First, the compatibility of different aryl aldehydes and primary amines in these multicomponent heterocyclization was examined. To our delight, a wide range of alkyl groups in the case of aryl aldehydes 2{1–26}, including methoxy, methyl, ethyl, propyl, butyl, allyl, benzyl and halogen, were well compatible in this transformation. The electronic effects of substituents had little influence on the cyclization. Note that a small excess of amines (1.1 eq.) was used in the interaction of aldehydes with a phenolic hydroxyl group. It should be mentioned that the substituted primary amines were also suitable for the transformation and synthesis of 2-alkyl-1-aryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones 4. But longer heating (up to 2 h) was required when using aldehydes with donor groups and shorter heating (15–20 min) when using aldehydes with acceptor groups. Methyl 4-(o-hydroxyphenyl)-2,4-dioxobutanoates with methyl, chloro and fluoro substituents were also tolerated in this one-pot multicomponent procedure, demonstrating the generality of the method. As a result, 223 out of 240 experiments allowed us to obtain the target 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones (92% success rate) with good purity (> 95% according to HPLC). In most cases, yields were in the 43–86% range, and for more than 50% of the representative set, the yield was more than 70%.
Chromones are widely used as excellent matrices for performing structural modifications allowing the synthesis of a wide range of compounds with different pharmacological profiles, which is certainly interesting for drug discovery [44,45,46,47,48]. Thus, the incorporation of pyrazole motif into the heterocyclic systems has led to important scaffolds attracted the attention of both industrial and medicinal chemists [44]. To demonstrate further the synthetic utility of the obtained 2-alkyl-1-aryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones 4, we developed ring-opening strategy with hydrazine hydrate, provides a wide scope of differently substituted pyrrolopyrazolones.
Initially, the recyclization of 2-methyl-1-phenyl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione 4{3–1-2} with hydrazine hydrate was examined (Scheme 4). We first tested the known literature method used for the recyclization of benzopyran-4-ones with hydrazine hydrate [17]. For compound 4{3–1-2} as a model compound, it was found that recyclization under the action of hydrazine hydrate proceeded in EtOH at different molar ratios of the starting reagents (from 1: 1 to 1: 3); however, the yields of the target product were rather low—up to 18% when the reaction was carried out at room temperature and not exceeding 30% at 80 °C. To increase the yield of the final product, an optimization of the reaction conditions was carried out. We varied the solvent, temperature, ratio of the reagents. The results obtained from the optimization are shown in Table 2. It should be noted that the yield of the target product 5{3–1-2} increased, and the reaction completion time decreased with an increase in the reaction temperature in both EtOH and dioxane, and with an increase in the molar ratio of the starting reagents (Entry 1–3 and 5–7). However, the reaction with the molar ratio of 1: 7 gave the product with a lower yield, and a complex mixture of unidentified products containing pyrazolecarboxylic acid hydrazide (Entry 4 and 8) was observed in the reaction mixture. Fairly efficient reaction conditions were found to be the use of the starting reagents in a molar ratio of 1: 5 in dioxane at 40 °C (Entry 7). An increase in the reaction temperature to 80 °C resulted in the lower yield (56%) of the product 5{3–1-2} in 4–6 h (Entry 9) due to the formation of a complex mixture of unidentified products. Finally, the product 5{3–1-2} was obtained with 78% yield when the reaction mixture was kept in dioxane at 80 °C for 20 h (Entry 10); notably, despite a noticeable amount of intermediate products being observed in the 1H NMR spectra of the reaction mixture after 4 and 12 h, only one key product was detected after 20 h.
Under the optimized conditions, a series of substituted 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5) were synthesized in a 20-mL glass vial using 1:5 molar ratio of chromeno[2,3-c]pyrrole-3,9-diones and hydrazine hydrate in dioxane by operationally simple procedure (Scheme 5). The wide availability of 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones makes the developed synthetic protocol a powerful tool for achieving a great variety of final products—4,5-dihydropyrrolo[3,4c]pyrazol-6(1H)-ones 5.
As a result, 223 experiments allowed us to obtain the target 4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones (100% success rate) and with good purity (> 95% according HPLC). In most cases, yields were in the 72–94% range, and for 50% of the representative set, the yield was more than 80%.
The correlation of proton signals in 1H NMR spectra of chromeno[2,3-c]pyrrole-3,9-diones can be in most cases performed quite easily based on signal multiplicity, but the appearance of the 13C NMR spectra was less predictable. The use of two-dimensional NMR spectroscopy methods on substance 4{3–1-2} made it possible to not only confirm the shown structure, but also to accurately identify the range of characteristic signals of the chromeno[2,3-c]pyrrole-3,9-dione system in 13C NMR spectra. The signal of γ-pyrone carbonyl group’s carbon is observed in the weakest field (more than 170 ppm); the carbon signal of the pyrrolone carbonyl group is observed in the field around 160 pm which is characteristic for amide groups. The carbon signal of the methine fragment of pyrrolone in the studied substance was slightly more than 60 ppm, although the spectra of some other derivatives, especially those with alkoxy groups, show other signals in the same region. For two-dimensional NMR experiments, CF3COOD was used because 4{3–1-2} is significantly higher solvable in it than in DMSO-d6; however, a comparison with the spectra taken in DMSO-d6 shows insignificant difference in chemical shifts. Figure 3 shows the assignments and the most important HMBC correlations (black doted) and NOESY correlations (red) for 4{3–1-2}.
As expected, 1H NMR spectra in DMSO-d6 show a significant high-field shift of protons of 2-hydroxyphenyl moiety of 4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones (5) compared to protons of chromone fragment of chromeno[2,3-c]pyrrole-3,9-diones (4). With no other substituents present, signals of the corresponding protons are located at 7.5–8.1 ppm for products 4 and at 6.7–7.5 ppm for products 5. However, 13C NMR spectra in DMSO-d6 of compounds 5 do not allow to identify carbon atoms of the pyrazole ring because of quick tautomeric transformations of pyrazole, and due to the weak intensity of the most low-field carbon atom of the pyrrolone carbonyl group, located at around 162 ppm. In particular, in the spectra of 5{3–1-2} it was only found due to correlations with the signals of the methine and N-methyl fragments. Assignment of pyrazole ring carbon atoms is possible when CF3COOD is used as a solvent; however, protonation causes wider signals in 1H NMR spectra. As an example, the product 5{3–1-2}’s 1H and 13C NMR spectral data in DMSO-d6 and CF3COOD is provided in the experimental part. Figure 4 shows the assignments and the most important HMBC correlations (black doted) and NOESY correlations (red) for 5{3–1-2}.
The IR spectra of products 4 contain fairly intense absorption bonds of carbonyl groups at 1717–1701 cm–1 and 1668–1647 cm–1, as well as rather intensive absorption of the unsaturated fragments conjugated with them, at approximately 1610 cm–1.
The higher-frequency absorption band is associated, evidently, with the carbonyl group of the chromone fragment, since there is no absorption in this region in the IR spectra of the recyclization products 5. At the same time, the amide band in the 1660–1690 cm–1 region remains very intense, partially overlapping with the absorption band of conjugated multiple bonds (approximately 1620 cm–1). The high acidity of the O–H and N–H bonds in pyrrolopyrazoles 5 causes a very broad absorption in the 3500–2500 cm–1 range.
Conclusion
In conclusion, we have developed a new one-pot version of a multicomponent reaction for the synthesis of libraries of functional 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones based on the interaction of methyl 4-(o-hydroxyphenyl)-2,4-dioxobutanoates, aryl aldehydes and primary amines. The possibility of using the obtained 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones for the synthesis of functional 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones was demonstrated. This protocol was found to be compatible with a wide range of substituents and paves the way for the practical synthesis of title compounds with a broad range of substituents under mild condition. The two synthetic techniques developed by this work are of interest for the synthesis of complex heterocyclic structures with possible biological activity. The resulting two libraries of compounds—1,2-dihydrochromeno-[2,3-c]pyrrole-3,9-diones (223 examples) and 3-(2-hydroxyphenyl)-4,5-dihydro-pyrrolo[3,4-c]pyrazol-6(1H)-ones (223 examples)—represent a real opportunity for the discovery of new drug candidates. Our group is currently investigating these potential applications, and the results will be published in due course.
Experimental
General remarks
The solvents were purified according to the standard procedures. All materials were purchased from commercial sources and used without further purification. Reaction flow and identity of obtained compounds were controlled with TLC on Merck F254 plates using chloroform: methanol (9:1, v/v) system as eluents. The success rate was calculated as the number of successful experiments divided by the total number of experiments. 1H NMR spectra were recorded on a Varian VXR-300 spectrometer (300 MHz) or Bruker 170 spectrometer (500 MHz), and 13C NMR spectra were recorded at Bruker 170 spectrometer (125 MHz) spectra in DMSO-d6 or CF3COOD, or CDCl3 solution. Chemical shifts are reported in ppm downfield from TMS as internal standards. Mass spectra were recorded on an LC–MS instrument with chemical ionization (CI). LC–MS data were acquired on an Agilent 1200 HPLC system equipped with DAD/ELSD/LCMS-6120 diode matrix and mass-selective detector. Melting points were measured on a MPA100 OptiMelt automated melting point system.
Combustion elemental analysis was performed by hand in the V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry analytical laboratory. The carbon and hydrogen contents were determined using the Pregl gravimetric method, nitrogen—using the Duma's gasometrical micromethod, sulfur—by the Scheininger titrimetric method, chlorine—by the mercurometric method.
Experimental Section describes 25 compounds selected in random manner, which corresponds to generally accepted approaches in combinatorial chemistry (according to ACS standards).
General procedure for the synthesis of 1-aryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones 4
To a solution of 0.01–0.011 mol of aryl aldehyde 2{1–26} in 10–15 mL of dry EtOH, 0.01–0.011 mol of primary amine 3{1–27} was added at room temperature and mixture was stirred for 0.3 h. Then, 0.01 mol of methyl o-hydroxybenzoylpyruvate 1{1–9} was added. The mixture was heated at 40 °C for 15–20 min, and 1 mL acetic acid was added. The resulting mixture was refluxed at 80 °C for 20 h; the reaction progress was monitored with TLC. After the completion of the reaction, the mixture was allowed to cool down to rt, and precipitate was filtered off (in most cases) or concentrated under vacuum and the residues were purified by crystallized from ethanol.
2-(Pyridin-4-ylmethyl)-1-(p-tolyl)-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{1–2-23}) Yield: 70%; mp 220–223 °C; IR (KBr): 2902, 1715 (C = O), 1656 (C = O), 1611, 1462, 1403, 1273, 1208, 1174, 894, 860, 822, 759, 556, 534 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.45 (d, J = 5.8 Hz, 2H), 8.03 (d, J = 8.3 Hz, 1H), 7.95–7.85 (m, 2H), 7.55 (ddd, J = 8.0, 5.6, 2.6 Hz, 1H), 7.23–7.15 (m, 4H), 7.13 (d, J = 7.9 Hz, 2H), 5.57 (s, 1H), 4.85 (d, J = 16.4 Hz, 1H), 4.03 (d, J = 16.4 Hz), 2H, 2.27 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 173.1, 162.2, 156.5, 155.0, 150.1, 145.8, 138.8, 135.5, 130.9, 129.9, 128.6, 128.0, 126.7, 125.7, 125.4, 122.9, 119.7, 60.2, 43.5, 21.2. APSI MS: 383.2 (M+ + 1). Anal. Calcd for C24H18N2O3: C, 75.38; H, 4.74; N, 7.33%. Found: C, 75.35; H, 4.79; N, 7.39%.
1-(4-(Allyloxy)phenyl)-2-methyl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{1–14-2}) Yield: 66%; mp 202–205 °C; IR (KBr): 2919, 1711 (C = O), 1657 (C = O), 1609, 1514, 1460, 1419, 1391, 1258, 1177, 1098, 896, 831, 758, 690, 565 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.03 (d, J = 7.9 Hz, 1H), 7.92–7.81 (m, 2H), 7.54 (t, J = 7.1 Hz, 1H), 7.26 (d, J = 6.8 Hz, 2H), 6.94 (d, J = 6.8 Hz, 2H), 6.10–5.96 (m, 1H), 5.56 (s, 1H), 5.39 (d, J = 17.3 Hz, 1H), 5.24 (d, J = 10.4 Hz, 1H), 4.59–4.51 (m, 2H), 2.79 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 161.5, 158.9, 156.4, 155.4, 135.3, 134.1, 129.6, 127.6, 126.6, 126.2, 125.7, 125.4, 119.7, 118.0, 115.4, 68.7, 60.9, 27.7. APSI MS: 348.2 (M+ + 1). Anal. Calcd for C21H17NO4: C, 72.61; H, 4.93; N, 4.03%. Found: C, 72.64; H, 4.90; N, 4.12%.
1-(2-Fluorophenyl)-2-propyl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{1–20-4}) Yield: 67%; mp 210–215 °C; IR (KBr): 2934, 1706 (C = O), 1655 (C = O), 1610, 1489, 1408, 1288, 1225, 1173, 1106, 757, 689 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.05 (d, J = 7.9 Hz, 1H), 7.94–7.82 (m, 2H), 7.55 (t, J = 7.3 Hz, 1H), 7.47–7.33 (m, 2H), 7.30–7.14 (m, 2H), 5.90 (s, 1H), 3.67–3.53 (m, 1H), 2.85 (dt, J = 13.7, 6.7 Hz, 1H), 1.61–1.34 (m, 2H), 0.80 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, DMSO-d6): δ 172.9, 161.7, 161.4 (d, J = 247.2 Hz), 156.4, 155.4, 135.4, 131.4 (d, J = 8.5 Hz), 126.9, 126.7, 125.7, 125.5 (d, J = 3.2 Hz), 125.1, 121.2 (d, J = 11.8 Hz), 119.7, 116.3 (d, J = 21.4 Hz), 42.6, 21.4, 11.5. APSI MS: 338.0 (M+ + 1). Anal. Calcd for C20H16FNO3: C, 71.21; H, 4.78; N, 4.15%. Found: C, 71.24; H, 4.75; N, 4.26%.
1-(3,4-Dichlorophenyl)-2-(1,1-dioxidotetrahydrothiophen-3-yl)-1,2-dihydro-chromeno[2,3-c]pyrrole-3,9-dione (4{1–24-27}) Yield: 71%; mp 200–202 °C; IR (KBr): 3558, 2951, 1717 (C = O), 1668 (C = O), 1608, 1463, 1402, 1365, 1313, 1267, 1203, 1122, 755, 570 cm−1. Two diastereomers. 1H NMR (400 MHz, DMSO-d6) δ 8.01 (d, J = 7.7 Hz, 1H), 7.93–7.84 (m, 3H), 7.63 (dd, J = 8.3, 5.2 Hz, 1H), 7.59–7.48 (m, 2H), 5.93 (s, 0.5H), 5.86 (s, 0.5H), 4.54–4.41 (m, 0.5H), 4.37–4.23 (m, 0.5H), 3.61 (dd, J = 12.9, 10.6 Hz, 0.5H), 3.46–3.25 (m, 2H with H2O), 3.19–3.04 (m, 1.5H), 2.77–2.63 (m, 0.5H), 2.36–2.18 (m, 1H), 2.13–2.01 (m, 0.5H). 13C NMR (100 MHz, DMSO-d6): δ 177.7, 177.7, 167.2, 167.1, 161.1, 161.1, 159.8, 159.5, 140.9, 140. 6, 140.3, 136.7, 136.7, 136.6, 136.5, 136.1, 136.1, 135.8, 135.7, 133.8, 133.6, 132.0, 131.8, 131.5, 131.4, 130.4, 130.0, 124.4, 64.3, 63.5, 56.3, 56.3, 56.2, 55.3, 55.0, 31.7, 31.5. APSI MS: 463.8 (M+ + 1). Anal. Calcd for C21H15Cl2NO5S: C, 54.32; H, 3.26; Cl, 15.27; N, 3.02; S, 6.90%. Found: C, 54.34; H, 3.29; Cl, 15.36; N, 3.13; S, 6.98%.
2,7-Dimethyl-1-phenyl-1H-chromeno[2,3-c]pyrrole-3,9-dione (4{3–1-2}) Yield: 76%; mp 242–244 °C; IR (KBr): 2921, 1710 (C = O), 1651 (C = O), 1614, 1477, 1422, 1386, 1276, 1255, 1212, 1137, 1096, 835, 703 cm−1. 1H NMR (500 MHz, CF3COOD) δ 8.13 (s, 1H), 7.84 (br. d, J = 8.3 Hz, 1H), 7.73 (d, J = 8.6 Hz, 1H), 7.53–7.41 (m, 3H), 7.33 (d, J = 5.9 Hz, 2H), 5.73 (s, 1H), 3.15 (s, 3H), 2.56 (s, 3H). 13C NMR (125 MHz, CF3COOD, APT): 175.9 (C), 163.4 (C), 155.3 (C), 155.0 (C), 138.3 (C), 137.4 (CH), 129.7 (CH), 129.5 (C), 128.9 (CH), 126.9 (CH), 126.6 (C), 124.4 (CH), 123.2 (C), 117.9 (CH), 63.8 (CH), 26.8 (CH3), 18.8 (CH3). APSI MS: 306 (M+ + 1). Anal. Calcd for C19H17NO3: C, 74.74; H, 4.95; N, 4.59%. Found: C, 74.84; H, 5.03; N, 4.53%.
2-Allyl-1-(4-ethylphenyl)-7-methyl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{3–3-6}) Yield: 43%; mp 235–237 °C; IR (KBr): 2965, 1709 (C = O), 1652 (C = O), 1615, 1477, 1408, 1279, 1211, 1138, 836 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.81 (s, 1H), 7.74–7.63 (m, 2H), 7.20 (s, 4H), 5.80–5.66 (m, 1H), 5.57 (s, 1H), 5.16–5.03 (m, 1H), 4.30 (dd, J = 15.7, 3.3 Hz, 1H), 3.41 (dd, J = 15.4, 6.0 Hz, 1H), 2.61 (q, J = 7.5 Hz, 2H), 2.42 (s, 3H), 1.18 (t, J = 7.5 Hz, 3H). 13C NMR (100 MHz, DMSO-d6): δ 173.0, 161.4, 154.9, 154.6, 144.7, 136.3, 132.9, 131.4, 128.6, 128.4, 127.6, 125.0, 124.9, 119.3, 118.2, 59.5, 43.0, 28.3, 20.8, 15.8. APSI MS: 360.0 (M+ + 1). Anal. Calcd for C23H21NO3: C, 76.86; H, 5.89; N, 3.90%. Found: C, 76.89; H, 5.86; N, 3.96%.
2-(2-Hydroxyethyl)-5,7-dimethyl-1-(3,4,5-trimethoxyphenyl)-1,2-dihydrochromeno-[2,3-c]pyrrole-3,9-dione (4{4–19-7}) Yield: 52%; mp 195–197 °C; IR (KBr): 3371, 2936, 1711, 1593, 1505, 1462, 1422, 1355, 1256, 1231, 1122, 1073, 1000, 823, 766, 705, 569 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.63 (s, 1H), 7.55 (s, 1H), 6.67 (s, 2H), 5.67 (s, 1H), 4.86 (br. s, 1H), 3.81–3.68 (m, 7H), 3.65 (s, 3H), 3.57–3.44 (m, 2H), 2.92–2.81 (m, 1H), 2.49 (s, 3H), 2.37 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 173.2, 161.9, 155.0, 153.5, 153.1, 137.9, 137.0, 135.5, 130.1, 128.3, 126.9, 125.0, 122.7, 105.8, 60.7, 60.4, 58.9, 56.5, 43.2, 20.8, 15.6. APSI MS: 440.1 (M+ + 1). Anal. Calcd for C24H25NO7: C, 65.59; H, 5.73; N, 3.19%. Found: C, 65.56; H, 5.76; N, 3.27%.
1-(4-(Allyloxy)-3-methoxyphenyl)-2-(2-methoxyethyl)-6,7-dimethyl-1,2-dihydro-chromeno[2,3-c]pyrrole-3,9-dione (4{5–17-9}) Yield: 55%; mp 166–169 °C; IR (KBr): 2924, 1712 (C = O), 1659 (C = O), 1622, 1517, 1465, 1426, 1262, 1230, 1143, 1013 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.71 (br. s, 1H), 7.59 (br. s, 1H), 6.92 (d, J = 7.3 Hz, 1H), 6.88 (s, 1H), 6.09–5.95 (m, 1H), 5.63 (s, 1H), 5.38 (d, J = 16.9 Hz, 1H), 5.24 (d, J = 9.8 Hz, 1H), 4.54 (s, 2H), 3.84 (d, J = 13.5 Hz, 1H), 3.69 (s, 3H), 3.40 (m, in H2O), 3.21 (s), 2.91 (d, J = 14.0 Hz, 1H), 2.37 (s, 3H), 2.29 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 172.8, 161.7, 154.8, 154.6, 149.6, 148.3, 145.5, 135.6, 134.2, 127.5, 126.7, 125.1, 123.1, 121.1, 119.3, 118.0, 113.7, 111.8, 69.6, 69.3, 60.2, 58.4, 56.1, 20.3, 19.3. APSI MS: 450.2 (M+ + 1). Anal. Calcd for C26H27NO6: C, 69.47; H, 6.05; N, 3.12%. Found: C, 69.45; H, 6.07; N, 3.25%.
7-Fluoro-2-(4-methoxyphenethyl)-1-(3-propoxyphenyl)-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{7–7-25}) Yield: 58%; mp 170–172 °C; IR (KBr): 2911, 1713 (C = O), 1656 (C = O), 1620, 1517, 1477, 1401, 1252, 1134 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.96 (dd, J = 9.2, 4.2 Hz, 1H), 7.81–7.73 (m, 1H), 7.70 (dd, J = 8.3, 3.0 Hz, 1H), 7.27 (t, J = 7.8 Hz, 1H), 7.06 (d, J = 8.5 Hz, 2H), 6.94–6.84 (m, 3H), 6.82 (d, J = 8.5 Hz, 2H), 5.54 (s, 1H), 3.94–3.80 (m, 3H), 3.71 (s, 3H), 3.03–2.90 (m, 1H), 2.86–2.75 (m, 1H), 2.68–2.58 (m, 1H), 1.71 (dt, J = 13.9, 6.9 Hz, 2H), 0.96 (t, J = 7.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6): δ 172.3, 160.7, 160.27 (d, J = 213.6 Hz), 158.7, 158.3, 155.7, 152.8, 135.7, 130.7, 130.3, 130.01, 126.6 (d, J = 7.0 Hz), 126.5, 123.2 (d, J = 23.6 Hz), 122.4 (d, J = 8.2 Hz), 120.4, 115.1, 114.6, 114.3, 110.5 (d, J = 20.9 Hz), 69.4, 59.8, 55.4, 42.4, 22.5, 10.8. APSI MS: 488.0 (M+ + 1). Anal. Calcd for C29H26FNO5: C, 71.45; H, 5.38; N, 2.87%. Found: C, 71.47; H, 5.40; N, 2.94%.
7-Chloro-1-(4-hydroxyphenyl)-2-phenethyl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione (4{8–11-24}) Yield: 72%; mp > 295 °C; IR (KBr): 3386, 1701 (C = O), 1647 (C = O), 1605, 1517, 1451, 1417, 1253, 1225, 1171, 1113, 840, 696 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.53 (s, 1H), 7.95–7.89 (m, 3H), 7.27 (t, J = 7.3 Hz, 2H), 7.20 (t, J = 6.8 Hz, 1H), 7.13 (dd, J = 13.5, 7.9 Hz, 4H), 6.75 (d, J = 8.4 Hz, 2H), 5.46 (s, 1H), 3.90–3.78 (m, 1H), 3.03–2.92 (m, 1H), 2.91–2.80 (m, 1H), 2.72–2.60 (m, 1H). 13C NMR (100 MHz, DMSO-d6): δ 171.9, 160.9, 158.3, 155.4, 155.0, 138.8, 135.1, 131.0, 129.7, 129.0, 128.9, 127.5, 126.9, 126.5, 124.65, 123.7, 122.2, 116.0, 59.5, 42.0, 34.1. APSI MS: 432.0 (M+ + 1). Anal. Calcd for C25H18ClNO4: C, 69.53; H, 4.20; Cl, 8.21; N, 3.24%. Found: C, 69.51; H, 4.23; Cl, 8.29; N, 3.28%.
7-Chloro-2-(furan-2-ylmethyl)-1-(3-hydroxyphenyl)-6-methyl-1,2-dihydrochromeno-[2,3-c]pyrrole-3,9-dione (4{9–5-21}) Yield: 62%; mp 276–279 °C; IR (KBr): 3330, 1694, 1659 (C = O), 1615, 1487, 1428, 1268, 1208, 1146, 691 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.51 (br. s, 1H), 7.94 (s, 1H), 7.90 (s, 1H), 7.59 (s, 1H), 7.16 (t, J = 7.8 Hz, 1H), 6.74 (m, 2H), 6.68 (m, 1H), 6.39 (dd, J = 3.0, 1.9 Hz, 1H), 6.25 (d, J = 3.0 Hz, 1H), 5.38 (s, 1H), 4.92 (d, J = 15.9 Hz, 1H), 3.90 (d, J = 15.9 Hz, 1H), 2.48 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 171.8, 161.1, 158.1, 154.8, 154.7, 149.6, 144.0, 143.5, 134.9, 131.9, 130.3, 127.6, 124.8, 124.5, 121.8, 118.9, 116.3, 115.1, 111.0, 109.2, 59.8, 37.1, 20.6. APSI MS: 422.0 (M+ + 1). Anal. Calcd for C23H16ClNO5: C, 65.49; H, 3.82; Cl, 8.40; N, 3.32%. Found: C, 65.45; H, 3.80; Cl, 8.48; N, 3.40%.
General procedure for the synthesis of 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo-[3,4-c]pyrazol-6(1H)-ones 5
Solution of 0.01 mol of chromeno[2,3-c]pyrrole-3,9-dione 4 and 0.05 mol of hydrazine hydrate in dry dioxane (10 mL) in a 20-mL glass vial was stirred at 80 °C for 15–20 h. After the completion of the reaction (reaction progress was monitored with TLC), the reaction mixture was allowed to cool down to rt, diluted with distil H2O (5 mL); the precipitate was filtered off and recrystallized from ethanol (5–7 mL).
*The exact position of the C = O and pyrazole signals cannot be established for certain compounds due to their low intensity.
3-(2-Hydroxyphenyl)-5-methyl-4-phenyl-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{1–1-2}) Yield: 94%; mp > 290 °C; IR (KBr): 3261–2414 (OH, NH), 1674, 1543, 1454, 1401, 1248, 826, 744, 695, 636 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.33–7.20 (m, 4H), 7.16 (d, J = 7.1 Hz, 2H), 7.10 (t, J = 7.7 Hz, 1H), 6.89 (d, J = 8.1 Hz, 1H), 6.71 (t, J = 7.4 Hz, 1H), 5.68 (s, 1H), 2.73 (s, 3H); OH and NH exchanged with H2O. 13C NMR (100 MHz, DMSO-d6): δ 162.6, 154.7, 150.3, 137.6, 134.5, 130.0, 129.2, 128.8, 128.1, 126.7, 119.5, 116.6, 115.7, 62.2, 27.9. APSI MS: 306.2 (M+ + 1). Anal. Calcd for C18H15N3O2: C, 70.81; H, 4.95; N, 13.76%. Found: C, 70.80; H, 4.92; N, 13.81%.
3-(2-Hydroxyphenyl)-5-(3-isopropoxypropyl)-4-(p-tolyl)-4,5-dihydropyrrolo[3,4-c]-pyrazol-6(1H)-one (5{1–2-12}) Yield: 75%; mp 198–200 °C; IR (KBr): 3468–2535 (OH, NH), 1678, 1543, 1464, 1260, 1129, 1090, 820, 760, 700, 638 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.35 (br. s, 1H), 10.40 (br. s, 1H), 7.25 (d, J = 7.6 Hz, 1H), 7.13–7.01 (m, 5H), 6.88 (d, J = 8.1 Hz, 1H), 6.71 (t, J = 7.5 Hz, 1H), 5.75 (s, 1H), 3.65 (dt, J = 14.4, 7.4 Hz, 1H), 3.50–3.39 (m, 1H), 3.30 (t, J = 6.0 Hz, 2H), 2.73–2.66 (m, 1H), 2.21 (s, 3H), 1.77–1.53 (m, 2H), 1.02 (dd, J = 5.9, 3.0 Hz, 6H). 13C NMR (100 MHz, DMSO-d6): δ 137.9, 130.9, 129.8, 128.8, 128.1, 119.5, 116.5, 71.0, 65.3, 60.1, 38.1, 28.8, 22.5, 21.2; *. APSI MS: 406.2 (M+ + 1). Anal. Calcd for C24H27N3O3: C, 71.09; H, 6.71; N, 10.36%. Found: C, 71.05; H, 6.73; N, 10.40%.
3-(2-Hydroxyphenyl)-4-(3-(pentyloxy)phenyl)-5-propyl-4,5-dihydropyrrolo[3,4-c]-pyrazol-6(1H)-one (5{1–8-4}) Yield: 83%; mp 242–244 °C; IR (KBr): 3339–2720 (OH, NH), 1689, 1583, 1533, 1462, 1427, 1284, 1231, 1091, 1011, 965, 818, 741, 700, 639 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.40 (br. s, 1H), 10.40 (br. s, 1H), 7.26 (d, J = 7.7 Hz, 1H), 7.18 (t, J = 7.9 Hz, 1H), 7.11 (t, J = 7.7 Hz, 1H), 6.89 (d, J = 8.1 Hz, 1H), 6.79 (d, J = 7.7 Hz, 1H), 6.76–6.67 (m, 3H), 5.74 (s, 1H), 3.91–3.77 (m, 2H), 3.63–3.50 (m, 1H), 2.71–2.59 (m, 1H), 1.69–1.58 (m, 2H), 1.56–1.46 (m, 1H), 1.46–1.37 (m, 1H), 1.37–1.24 (m, 4H), 0.86 (t, J = 6.9 Hz, 3H), 0.79 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, DMSO-d6)*: δ 162.8, 159.2, 154.5, 130.3, 130.0, 128.9, 126.6, 119.8, 119.4, 116.4, 114.4, 114.3, 67.8, 59.9, 42.2, 28.7, 28.1, 22.3, 21.5, 14.3, 11.7. APSI MS: 420.2 (M+ + 1). Anal. Calcd for C25H29N3O3: C, 71.57; H, 6.97; N, 10.02%. Found: C, 71.55; H, 6.96; N, 10.13%.
5-(Benzo[d][1,3]dioxol-5-ylmethyl)-3-(2-hydroxyphenyl)-4-(4-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{1–11-18}) Yield: 90%; mp 280–282 °C; IR (KBr): 3532–2545 (OH, NH), 1664, 1600, 1515, 1473, 1337, 1246, 1209, 1086, 1039, 1000, 926, 841, 804, 740, 670, 564 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.44 (br. s, 1H), 10.32 (br. s, 1H), 9.47 (br. s, 1H), 7.22 (d, J = 7.6 Hz, 1H), 7.10–7.06 (m, 1H), 6.87–6.83 (m, 4H), 6.69–6.62 (m, 5H), 5.99 (d, J = 6.0 Hz, 2H), 5.42 (s, 1H), 4.86 (d, J = 15.2 Hz, 1H), 3.54 (d, J = 15.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6)*: δ 157.7, 147.9, 146.8, 131.8, 130.0, 129.4, 128.8, 121.4, 119.5, 116.4, 115.9, 108.7, 108.5, 101.4, 59.5, 43.6. APSI MS: 442,2 (M+ + 1). Anal. Calcd for C25H19N3O5: C, 68.02; H, 4.34; N, 9.52%. Found: C, 68.00; H, 4.36; N, 9.60%.
4-(4-(Benzyloxy)phenyl)-3-(2-hydroxyphenyl)-5-(3-methoxypropyl)-4,5-dihydro-pyrrolo[3,4-c]pyrazol-6(1H)-one (5{1–15-10}) Yield: 72%; mp 173–175 °C; IR (KBr): 3296–2621 (OH, NH), 1668, 1515, 1462, 1250, 1109, 744, 691, 636 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.40 (br. s, 1H), 10.40 (br. s, 1H), 7.46–7.28 (m, 5H), 7.23 (d, J = 7.5 Hz, 1H), 7.16–7.06 (m, 3H), 6.94 (d, J = 8.5 Hz, 2H), 6.89 (d, J = 8.1 Hz, 1H), 6.71 (t, J = 7.3 Hz, 1H), 5.74 (s, 1H), 5.01 (s, 2H), 3.68–3.55 (m, 1H), 3.31–3.23 (m, 2H), 3.18 (s, 3H), 2.79–2.66 (m, 1H), 1.80–1.67 (m, 1H), 1.67–1.54 (m, 1H). 13C NMR (100 MHz, DMSO-d6)*: δ 158.7, 137.3, 130.0, 129.5, 128.9, 128.3, 119.5, 116.5, 115.4, 70.0, 69.7, 59.9, 58.3, 38.0, 28.4. APSI MS: 470.2 (M+ + 1). Anal. Calcd for C28H27N3O4: C, 71.62; H, 5.80; N, 8.95%. Found: C, 71.63; H, 5.83; N, 8.99%.
4-(4-Hydroxy-3-methoxyphenyl)-3-(2-hydroxyphenyl)-5-propyl-4,5-dihydropyrrolo-[3,4-c]pyrazol-6(1H)-one (5{1–16-4}) Yield: 78%; mp 243–245 °C; IR (KBr): 3496–2797 (OH, NH), 1677, 1600, 1515, 1467, 1391, 1258, 1220, 1128, 1183, 1037, 741, 718 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.17 (d, J = 7.7 Hz, 1H), 7.05 (t, J = 7.7 Hz, 1H), 6.88 (d, J = 8.1 Hz, 1H), 6.76 (s, 1H), 6.70 (d, J = 8.1 Hz, 1H), 6.62 (m, 2H), 5.65 (s, 1H), 3.64 (s, 3H), 3.61–3.49 (m, 1H), 2.76–2.64 (m, 1H), 1.57–1.33 (m, 2H), 0.79 (t, J = 7.3 Hz, 3H); OH and NH exchanged with H2O. 13C NMR (100 MHz, DMSO-d6): δ 162.3, 156.4, 149.0, 148.1, 146.9, 137.5, 129.4, 128.6, 128.2, 127.0, 120.6, 118.5, 116.8, 116.3, 116.1, 112.2, 59.9, 56.1, 42.1, 21.6, 11.7. APSI MS: 380.2 (M+ + 1). Anal. Calcd for C21H21N3O4: C, 66.48; H, 5.58; N, 11.08%. Found: C, 66.50; H, 5.55; N, 11.12%.
3-(2-Hydroxyphenyl)-5-(pyridin-4-ylmethyl)-4-(3,4,5-trimethoxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{1–19-23}) Yield: 81%; mp 280–282 °C; IR (KBr): 3451 (OH), 2940, 1689 (C = O), 1658 (C = O), 1596, 1467, 1425, 1263, 1249, 1201, 1129, 1006, 712 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.45 (d, J = 5.9 Hz, 2H), 7.30 (dd, J = 7.7, 1.5 Hz, 1H), 7.17–7.08 (m, 3H), 6.87 (d, J = 8.1 Hz, 1H), 6.74 (t, J = 7.6 Hz, 1H), 6.38 (s, 2H), 5.62 (s, 1H), 4.85 (d, J = 16.4 Hz, 1H), 4.15 (d, J = 16.4 Hz, 1H), 3.59 (s, 6H), 3.55 (s, 3H); OH and NH exchanged with H2O. 13C NMR (100 MHz, DMSO-d6)*: δ 154.7, 153.4, 150.1, 147.1, 137.5, 130.2, 129.1, 122.9, 119.5, 116.3, 105.2, 61.1, 60.3, 56.2, 43.9. APSI MS: 473.0 (M+ + 1). Anal. Calcd for C26H24N4O5: C, 66.09; H, 5.12; N, 11.86%. Found: C, 66.06; H, 5.10; N, 11.92%.
4-(4-Chlorophenyl)-5-(1,1-dioxidotetrahydrothiophen-3-yl)-3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{1–22-27}) Yield: 75%; mp 240–242 °C; IR (KBr): 3471–2631 (OH, NH), 1671, 1624, 1587, 1544, 1491, 1462, 1412, 1317, 1239, 1127, 1093, 833, 741, 683, 570, 521 cm−1. Two diastereomers. 1H NMR (400 MHz, DMSO-d6) δ 13.57 (br. s, 1H), 10.38 (br. s, 1H), 7.37–7.31 (m, 2H), 7.31–7.21 (m, 3H), 7.12 (t, J = 7.5 Hz, 1H), 6.89 (dd, J = 7.9, 3.4 Hz, 1H), 6.75 (td, J = 7.5 Hz, 3.2 Hz, 1H), 5.96 (s, 0.5H), 5.92 (s, 0.5H), 4.37–4.25 (m, 0.5H), 4.25–4.16 (m, 0.5H), 3.63–3.52 (m, 0.5H), 3.41 (dd, J = 12.7, 8.1 Hz, 0.5H), 3.34–3.23 (m, 1.5H), 3.13–3.00 (m, 1H), 2.89 (dd, J = 12.8, 8.1 Hz, 0.5H), 2.69–2.53 (m, 0.5H), 2.35 (td, J = 10.6, 7.8 Hz, 0.5H), 2.29–2.19 (m, 0.5H), 1.97–1.86 (m, 0.5H). 13C NMR (100 MHz, DMSO-d6)*: δ 154.6, 133.3, 133.2, 130.3, 130.1, 129.2, 128.7, 128.5, 119.5, 116.4, 61.0, 60.4, 51.8, 51.7, 51.7, 51.2, 50.7, 50.5, 26.9. APSI MS: 444.0 (M+ + 1). Anal. Calcd for C21H18ClN3O4S: C, 56.82; H, 4.09; Cl, 7.99; N, 9.47; S, 7.22%. Found: C, 56.80; H, 4.05; Cl, 7.90; N, 9.57; S, 7.28%.
5-(Furan-2-ylmethyl)-3-(2-hydroxyphenyl)-4-(4-(methylthio)phenyl)-4,5-dihydro-pyrrolo[3,4-c]pyrazol-6(1H)-one (5{1–25-21}) Yield: 73%; mp 242–244 °C; IR (KBr): 3339–2720 (OH, NH), 1689, 1619, 1583, 1533, 1494, 1462, 1427, 1372, 1231, 1091, 833, 818, 742, 700 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.50 (br. s, 1H), 10.50 (br. s, 1H), 7.60 (s, 1H), 7.30 (d, J = 7.7 Hz, 1H), 7.15 (d, J = 8.1 Hz, 2H), 7.10 (t, J = 7.3 Hz, 1H), 7.04 (d, J = 7.9 Hz, 2H), 6.84 (d, J = 8.1 Hz, 1H), 6.73 (t, J = 7.3 Hz, 1H), 6.41 (s, 1H), 6.24 (d, J = 2.5 Hz, 1H), 5.53 (s, 1H), 4.94 (d, J = 16.0 Hz, 1H), 3.67 (d, J = 16.0 Hz, 1H), 2.41 (s, 3H). 13C NMR (100 MHz, DMSO-d6)*: δ 154.8, 150.9, 143.3, 138.7, 130.2, 128.8, 128.7, 126.3, 119.6, 116.4, 111.0, 108.7, 60.0, 36.9, 14.7. APSI MS: 418.2 (M+ + 1). Anal. Calcd for C23H19N3O3S: C, 66.17; H, 4.59; N, 10.07; S, 7.68%. Found: C, 66.15; H, 4.56; N, 10.17; S, 7.78%.
3-(2-Hydroxy-5-methylphenyl)-5-methyl-4-phenyl-1,4-dihydropyrrolo[3,4-c]pyrazol-6-one (5{3–1-2}) Yield: 85%; mp 275–278 °C; IR (KBr): 3619–2817 (OH, NH), 1649, 1487, 1458, 1383, 1264, 1085, 805, 710 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.35 (t, J = 7.4 Hz, 2H), 7.30 (t, J = 7.0 Hz, 2H), 7.22 (d, J = 7.2 Hz, 2H), 6.96 (br. s, 1H), 6.92 (br. d, J = 8.2 Hz, 1H), 6.80 (d, J = 8.2 Hz, 1H), 5.70 (s, 1H), 2.76 (s, 3H), 2.08 (s, 3H), OH and NH exchanged with H2O. 1H NMR (400 MHz, CF3COOD) δ 8.84 (br. s, 1H), 8.67–8.66 (m, 1H), 8.52–8.50 (m, 1H), 8.30–8.24 (m, 2H), 7.21 (s, 1H), 4.43 (s, 3H), 3.37 (s, 3H). 13C NMR (100 MHz, DMSO-d6, APT)*: 162.3 (C), 152.5 (C), 137.7 (C), 130.4 (CH), 129.2 (CH), 129.1 (CH), 128.7 (CH), 128.2 (CH), 128.0 (C), 116.3 (CH), 115.3 (C), 62.1 (CH), 27.9 (CH3), 20.3 (CH3). 13C NMR (100 MHz, CF3COOD): δ 159.4, 153.4, 144.2, 142.9, 136.5, 133.4, 132.1, 131.3, 131.2, 131.1, 129.1, 129.0, 117.6, 110.2, 66.2, 29.2, 19.2. APSI MS: 320.4 (M+ + 1). Anal. Calcd for C19H17N3O2: C, 71.5; H, 5.4; N, 13.2%. Found: C, 71.3; H, 5.6; N, 13.4%.
3-(2-Hydroxy-5-methylphenyl)-4-(3-hydroxyphenyl)-5-(3-isopropoxypropyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{3–5-12}) Yield: 78%; mp 232–235 °C; IR (KBr): 3517–2484 (OH, NH), 1686, 1604, 1499, 1458, 1375, 1257, 1223, 1038, 1093, 826, 758, 686 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.27 (br. s, 1H), 10.21 (br. s, 1H), 9.40 (br. s, 1H), 7.13 (t, J = 7.5 Hz, 1H), 6.96 (s, 1H), 6.90 (d, J = 8.3 Hz, 1H), 6.83–6.71 (m, 2H), 6.66 (d, J = 7.6 Hz, 1H), 6.50 (s, 1H), 5.71 (s, 1H), 3.74–3.60 (m, 1H), 3.50–3.41 (m, 1H), 3.34–3.24 (m, 2H), 2.79–2.67 (m, 1H), 2.07 (s, 3H), 1.79–1.57 (m, 2H), 1.11–0.98 (m, 6H). 13C NMR (100 MHz, DMSO-d6)*: δ 158.1, 130.3, 130.1, 129.3, 128.0, 119.3, 116.2, 115.8, 114.3, 71.0, 65.3, 60.0, 38.2, 28.8, 22.4, 20.2. APSI MS: 422.2 (M+ + 1). Anal. Calcd for C24H27N3O4: C, 68.39; H, 6.46; N, 9.97%. Found: C, 68.36; H, 6.54; N, 9.90%.
4-(3-(Allyloxy)phenyl)-3-(2-hydroxy-5-methylphenyl)-5-(2-hydroxyethyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{3–9-7}) Yield: 72%; mp 229–231 °C; IR (KBr): 3431–2627 (OH, NH), 1663, 1583, 1489, 1460, 1274, 1079, 1041, 806, 766 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.32 (br. s, 1H), 10.19 (br. s, 1H), 7.21 (t, J = 8.0 Hz, 1H), 6.99 (s, 1H), 6.91 (d, J = 8.0 Hz, 1H), 6.88–6.75 (m, 3H), 6.71 (br. d, J = 6.0 Hz, 1H), 5.98 (ddd, J = 22.3, 10.5, 5.3 Hz, 1H), 5.84 (s, 1H), 5.33 (d, J = 17.3 Hz, 1H), 5.21 (d, J = 10.7 Hz, 1H), 4.82 (br. t, J = 4.6 Hz, 1H), 4.54–4.39 (m, 2H), 3.78–3.66 (m, 1H), 3.53 (td, J = 10.8, 5.2 Hz, 1H), 3.44 (td, J = 10.8, 5.6 Hz, 1H), 2.76–2.63 (m, 1H), 2.08 (s, 3H). 13C NMR (100 MHz, DMSO-d6)*: δ 158.7, 152.4, 133.9, 130.4, 130.3, 129.2, 127.9, 120.2, 117.9, 116.2, 115.0, 114.5, 68.6, 60.8, 59.4, 43.2, 20.2. APSI MS: 406.0 (M+ + 1). Anal. Calcd for C23H23N3O4: C, 68.13; H, 5.72; N, 10.36%. Found: C, 68.15; H, 5.75; N, 10.31%.
3-(2-Hydroxy-3,5-dimethylphenyl)-5-(2-hydroxyethyl)-4-(4-methoxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{4–12-7}) Yield: 81%; mp 192–194 °C; IR (KBr): 3428–2749 (OH, NH), 1661, 1511, 1483, 1391, 1241, 1174, 1063, 831, 784, 744, 696 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 14.20 (br. s, 1H), 10.65 (br. s, 1H), 7.20 (br. d, J = 6.4 Hz, 2H), 6.90 (d, J = 8.1 Hz, 2H), 6.80 (s, 1H), 6.74 (s, 1H), 5.89 (s, 1H), 4.83 (br. s, 1H), 3.78–3.60 (m, 4H), 3.60–3.49 (m, 1H), 3.49–3.39 (m, 1H), 2.74–2.62 (m, 1H), 2.13 (s, 3H), 2.02 (s, 3H). 13C NMR (100 MHz, DMSO-d6)*: δ 159.8, 131.6, 129.9, 126.7, 114.7, 60.2, 59.4, 55.6, 43.2, 20.2, 16.5. APSI MS: 394.0 (M+ + 1). Anal. Calcd for C22H23N3O4: C, 67.16; H, 5.89; N, 10.68%. Found: C, 67.14; H, 5.86; N, 10.73%.
5-(2-Chlorobenzyl)-4-(3-ethoxy-4-hydroxyphenyl)-3-(2-hydroxy-3,5-dimethyl-phenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{4–18-20}) Yield: 84%; mp 247–249 °C; IR (KBr): 3571–2597 (OH, NH), 1689, 1610, 1515, 1443, 1276, 1237, 1210, 1120, 1039, 825, 750, 617 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.47–7.39 (m, 1H), 7.35–7.24 (m, 2H), 7.18 (dd, J = 5.4, 3.9 Hz, 1H), 6.80 (d, J = 3.9 Hz, 2H), 6.74 (s, 1H), 6.69 (d, J = 8.1 Hz, 1H), 6.54 (d, J = 7.4 Hz, 1H), 5.58 (s, 1H), 4.91 (d, J = 16.3 Hz, 1H), 4.01 (d, J = 16.2 Hz, 1H), 3.86 (q, J = 6.9 Hz, 2H), 2.12 (s, 3H), 2.00 (s, 3H), 1.24 (t, J = 6.9 Hz, 3H), OH and NH exchanged with H2O. 13C NMR (100 MHz, DMSO-d6)*: δ 154.5, 151.0, 147.4, 147.1, 134.8, 132.5, 131.7, 129.8, 129.5, 127.8, 126.9, 125.2, 120.8, 116.2, 115.6, 114.0, 64.3, 60.4, 42.1, 20.2, 16.6, 15.0. APSI MS: 504.0 (M+ + 1). Anal. Calcd for C28H26ClN3O4: C, 66.73; H, 5.20; Cl, 7.03; N, 8.34%. Found: C, 66.71; H, 5.23; Cl, 7.13; N, 8.41%.
4-(4-Bromophenyl)-3-(2-hydroxy-3,5-dimethylphenyl)-5-(pyridin-3-ylmethyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{4–23-22}) Yield: 81%; mp 241–244 °C; IR (KBr): 3367–2426 (OH, NH), 1699, 1559, 1481, 1435, 1253, 1072, 1011, 835, 751, 712, 636 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.47 (d, J = 4.3 Hz, 1H), 8.36 (s, 1H), 7.58 (d, J = 7.7 Hz, 1H), 7.48 (d, J = 7.9 Hz, 2H), 7.33 (dd, J = 7.7, 4.8 Hz, 1H), 7.17 (br. s, 2H), 6.83–6.74 (m, 2H), 5.77 (br. s, 1H), 4.88 (d, J = 16.0 Hz, 1H), 3.91 (d, J = 16.0 Hz, 1H), 2.11 (s, 3H), 2.02 (s, 3H), OH and NH exchanged with H2O. 13C NMR (100 MHz, DMSO-d6)*: δ 149.5, 149.0, 136.0, 133.3, 132.2, 130.8, 126.4, 124.1, 59.6, 42.2, 20.2. APSI MS: 489.0 (M+ + 1). Anal. Calcd for C25H21BrN4O2: C, 61.36; H, 4.33; N, 11.45%. Found: C, 61.33; H, 4.30; N, 11.49%.
5-(4-Fluorobenzyl)-3-(2-hydroxy-4,5-dimethylphenyl)-4-(4-(methylthio)phenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{5–25-19}) Yield: 81%; mp 210–213 °C; IR (KBr): 3466–2483 (OH, NH), 1678, 1510, 1464, 1227, 1092, 1070, 825 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.25 (br. s, 1H), 9.88 (br. s, 1H), 7.28–6.99 (m, 8H), 6.93 (s, 1H), 6.63 (s, 1H), 5.50 (br. s, 1H), 4.91 (d, J = 15.2 Hz, 1H), 3.73 (br. d, J = 14.4 Hz, 1H), 2.42 (s, 3H), 2.07 (s, 3H), 1.98 (s, 3H). 13C NMR (100 MHz, DMSO-d6): δ 162.8, 161.8 (d, J = 243.3 Hz), 152.3, 149.7, 138.7, 138.4, 135.0, 134.2, 133.7, 130.1 (d, J = 8.2 Hz), 129.5, 128.9, 127.0, 126.3, 117.4, 115.78 (d, J = 21.4 Hz), 112.4, 59.6, 43.3, 19.7, 18.5, 14.7. APSI MS: 474.2 (M+ + 1). Anal. Calcd for C27H24FN3O2S: C, 68.48; H, 5.11; N, 8.87; S, 6.77%. Found: C, 68.45; H, 5.13; N, 8.81; S, 6.87%.
3-(5-Chloro-2-hydroxy-4-methylphenyl)-5-(2-chlorobenzyl)-4-(4-hydroxy-3- methoxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{9–16-20}) Yield: 79%; mp 242–244 °C; IR (KBr): 3371–2622 (OH, NH), 1679, 1515, 1454, 1264, 1228, 1171, 1141, 1031, 764 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.07 (br. s, 1H), 7.47–7.39 (m, 1H), 7.34–7.26 (m, 2H), 7.24 (s, 1H), 7.19–7.12 (m, 1H), 6.80 (s, 1H), 6.71–6.60 (m, 2H), 6.46 (d, J = 7.9 Hz, 1H), 5.55 (s, 1H), 4.93 (d, J = 16.0 Hz, 1H), 4.00 (d, J = 16.0 Hz, 1H), 3.64 (s, 3H), 2.18 (s, 3H); another OH and NH exchanged with H2O. 13C NMR (100 MHz, DMSO-d6)*: δ 147.9, 146.9, 137.0, 134.9, 132.5, 129.9, 129.8, 129.4, 128.5, 127.8, 123.5, 120.3, 118.6, 116.2, 112.4, 60.4, 55.9, 42.1, 19.9. APSI MS: 510.0 (M+ + 1). Anal. Calcd for C26H21Cl2N3O4: C, 61.19; H, 4.15; Cl, 13.89; N, 8.23%. Found: C, 61.16; H, 4.19; Cl, 13.97; N, 8.31%.
3-(5-Chloro-2-hydroxy-4-methylphenyl)-4-(3-chlorophenyl)-5-(3-hydroxypropyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-one (5{9–21-8}) Yield: 80%; mp 275–277 °C; IR (KBr): 3479–2513 (OH, NH), 1674, 1466, 1397, 1297, 1096, 1071, 704 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 13.45 (br. s, 1H), 10.55 (br. s, 1H), 7.37–7.32 (m, 2H), 7.30 (s, 1H), 7.25 (s, 1H), 7.13–7.05 (m, 1H), 6.83 (br. s, 1H), 5.85 (s, 1H), 4.47 (br. s, 1H), 3.67 (dt, J = 14.1, 7.2 Hz, 1H), 3.40–3.35 (m, 2H), 2.75 (ddd, J = 14.1, 8.2, 5.9 Hz, 1H), 2.19 (s, 3H), 1.72–1.60 (m, 1H), 1.60–1.48 (m, 1H). 13C NMR (100 MHz, DMSO-d6)*: δ 153.3, 137.1, 133.7, 131.2, 128.8, 128.2, 126.6, 123.5, 118.7, 59.6, 58.8, 38.2, 31.5, 19.9. APSI MS: 432.0 (M+ + 1). Anal. Calcd for C21H19Cl2N3O3: C, 58.35; H, 4.43; Cl, 16.40; N, 9.72%. Found: C, 58.32; H, 4.40; Cl, 16.48; N, 9.79%.
References
Welsch ME, Snyder SA, Stockwell BR (2010) Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol 14(3):347–361. https://doi.org/10.1016/j.cbpa.2010.02.018
Rodrigues T, Reker D, Schneider P, Schneider G (2016) Counting on natural products for drug design. Nature Chem 8(6):531–541. https://doi.org/10.1038/nchem.2479
Schreiber SL (2000) Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287(5460):1964–1969. https://doi.org/10.1126/science.287.5460.1964
Zhang L, Zheng M, Zhao F, Zhai Yu, Liu H (2014) Rapid generation of privileged substructure-based compound libraries with structural diversity and drug-likeness. ACS Comb Sci 16(4):184–191. https://doi.org/10.1021/co4001309
Pelish HE, Westwood NJ, Ya F, Kirchhausen T, Shair MD (2001) Use of biomimetic diversity-oriented synthesis to discover galanthamine-like molecules with biological properties beyond those of the natural product. J Am Chem Soc 123(27):6740–6741. https://doi.org/10.1021/ja016093h
Schreiber SL (2003) The small molecule approach to biology. Chem Eng News 81:51–61. http://portals.broadinstitute.org/chembio/lab_schreiber/pubs/pdffiles/331.pdf
Edwards AM, Howell JBL (2000) The chromones: history, chemistry and clinical development. A tribute to the work of Dr R. E. C. Altounyan. Clin Exp Allergy 30(6):756–774. https://doi.org/10.1046/j.1365-2222.2000.00879.x
Wilk W, Waldmann H, Kaiser M (2009) γ-Pyrone natural products – a privileged compound class provided by nature. Bioorg Med Chem 17(6):2304–2309. https://doi.org/10.1016/j.bmc.2008.11.001
Khadem Sh, Marles RJ (2012) Chromone and flavonoid alkaloids: occurrence and bioactivity. Molecules 17(1):191–206. https://doi.org/10.3390/molecules17010191
Silva CFM, Batista VF, Pinto DCGA, Silva AMS (2018) Challenges with chromone as a privileged scaffold in drug discovery. Exp Opinion Drug Disc 13(9):795–798. https://doi.org/10.1080/17460441.2018.1494720
Reis J, Gaspar A, Milhazes N, Borges F (2017) Chromone as a privileged scaffold in drug discovery: recent advances. J Med Chem 60(19):7941–7957. https://doi.org/10.1021/acs.jmedchem.6b01720
Gaspar A, Matos MJ, Garrido J, Uriarte Eu, Borges F (2014) Chromone: a valid scaffold in medicinal chemistry. Chem Rev 114(9):4960–4992. https://doi.org/10.1021/cr400265z
Keri RS, Budagumpi S, Pai RK, Balakrishna RG (2014) Chromones as a privileged scaffold in drug discovery: a review. Eur J Med Chem 78:340–374. https://doi.org/10.1016/j.ejmech.2014.03.047
Ya Z, Zhang X, Yao R, Wen YuCh, Huang J, Xu X (2016) 1,3-Dipolar Cycloaddition of alkyne-tethered N-tosylhydrazones: synthesis of fused polycyclic pyrazoles. J Org Chem 22:11072–11080. https://doi.org/10.1021/acs.joc.6b02076
Yadav R, Parvin T, Panday AK, Choudhury LH (2021) Synthesis of styryl-linked fused dihydropyridines by catalyst-free multicomponent reactions. Mol Divers. https://doi.org/10.1007/s11030-021-10216-4
Yavari I, Sheykhahmadi J (2021) TFA-mediated synthesis of functionalized pyrano[2,3-c]pyrazoles from pyrazol-3-ones, active carbonyl compounds and tert-BuOH. Mol Divers. https://doi.org/10.1007/s11030-021-10200-y
Santos CMM, Silva VLM, Silva AMS (2017) Synthesis of chromone-related pyrazole compounds. Molecules 22(10):1665. https://doi.org/10.3390/molecules22101665
Malets YS, Moskvina VS, Grygorenko OO, Brovarets VS (2019) Synthesis of azachromones and azachromanones. Chem Heterocycl Comp. https://doi.org/10.1007/s10593-019-02570-x
Miyake Y, Mochizuki M, Ito C, Itoigawa M, Osawa T (2008) Antioxidative pyranonigrins in rice mold starters and their suppressive effect on the expression of blood adhesion molecules. Biosci Biotechnol Biochem 72(6):1580–1585. https://doi.org/10.1271/bbb.80077
Hiort J, Maksimenka K, Reichert M, Perović-Ottstadt S, Lin WH, Wray V, Steube K, Schaumann K, Weber H, Proksch P, Ebel R, Müller WE, Bringmann G (2004) New natural products from the sponge-derived fungus Aspergillus niger. J Nat Prod 67(9):1532–1543. https://doi.org/10.1021/np030551d
Schlingmann G, Taniguchi T, He H, Bigelis R, Yang HY, Koehn FE, Carter GT, Berova N (2007) Reassessing the structure of Pyranonigrin. J Nat Prod 70(7):1180–1187. https://doi.org/10.1021/np070175n
Kishimoto S, Tsunematsu Y, Sato M, Watanabe K (2017) Elucidation of biosynthetic pathways of natural products. Chem Rec 17(11):1095–1108. https://doi.org/10.1002/tcr.201700015
Riko R, Nakamura H, Shindo K (2014) Studies on pyranonigrins–isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A. J Antibiot 67:179–181. https://doi.org/10.1038/ja.2013.91
Rao P, Shukla A, Parmar P, Rawal RM, Patel B, Saraf M, Goswami D (2020) Reckoning a fungal metabolite, Pyranonigrin A as a potential Main protease (Mpro) inhibitor of novel SARS-CoV-2 virus identified using docking and molecular dynamics simulation. Biophysical Chem. https://doi.org/10.1016/j.bpc.2020.106425
Sarabu R, inventor; Hoffmann-La Roche, applicant. 3-Oxo-3,9-dihydro-1H-chromeno[2,3-c]pyrroles as glucokinase activators. Canadian Patent Application CA2801168A1. 2011 Dec 22
Sidhu PS, Mosier PD, Zhou Q, Desai UR (2013) On scaffold hopping: challenges in the discovery of sulfated small molecules as mimetics of glycosaminoglycans. Bioorg Med Chem Lett 23(1):355–359. https://doi.org/10.1016/j.bmcl.2012.10.079
Vydzhak RN, Panchishin SY (2006) Simple synthesis of 1,2-diaryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones. Russ J General Chem 76:1681–1682. https://doi.org/10.1134/S1070363206100331
Vydzhak RN, Panchishin SY (2008) Synthesis of 2-alkyl-1-aryl-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-dione derivatives. Russ J General Chem 78:2391–2397. https://doi.org/10.1134/S1070363208120165
Vydzhak RN, Panchishin SY (2010) Synthesis of 1-aryl-2-[2-(dimethylamino)ethyl]-1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones and their analogs. Russ J General Chem 80:323–329. https://doi.org/10.1134/S1070363210020222
Vydzhak RN, Panchishin SY (2008) Synthesis of 2-phenyl-5,6-dihydropyrano[2,3-c]pyrrole-4,7-dione derivatives. Russ J General Chem 78:1641–1642. https://doi.org/10.1134/S1070363208080331
Ellis GP, Romney-Alexander TM (1985) Benzopyrones. Part 20. Synthesis of new tri- and tetracyclic chromen-4-ones. Chem Inform 16(18):206. https://doi.org/10.1002/chin.198518206
Vydzhak RN, Panchishin SY (2011) Synthesis of 1,2-dihydro-chromeno[2,3-c]pyrrole-3,9-diones spiro derivatives. Russ J General Chem 81:617–619. https://doi.org/10.1134/S1070363211030340
Vydzhak RN, Kachaeva MV, Pilyo SG, Moskvina VS, Shablykina OV, Kozytskiy AV, Brovarets VS (2020) Three-component cyclization as an approach to a combinatorial library of 2H-spiro-[chromeno[2,3-c]pyrrole-1,3’-indoline]-2’,3,9-triones. Ukr Bioorg Acta 15(1):26–33. https://doi.org/10.15407/bioorganica2020.01.026
Neo AG, Garrido L, Dнaz J, Marcaccini S, Marcos CF (2012) Furo[3,4-b]chromones, and not pyrano[3,4-b]chromones, are obtained by the reaction of 3-formylchromones with isocyanides. Synlett 23(15):2227–2230. https://doi.org/10.1055/s-0032-1317032
Panja SK, Maiti S, Banerjee S, Bandyopadhyay C (2010) One-pot synthesis of pyrano[3,4-b]chromones from chromone-3-carbaldehyde. Synlett 13:1909–1914. https://doi.org/10.1055/s-0030-1258496
Panja SK, Maiti S, Drew MGB, Bandyopadhyay C (2011) One-pot three component reaction involving cyclohexyl isocyanide for the synthesis of furo [3, 4-b] chromenes. J Chem Res. https://doi.org/10.3184/174751911X13015945166625
Teimouri MB (2011) Serendipitous stereoselective synthesis of brand-new fluorescent dyes: (1Z)-3-(alkylimino)-1-[(chromone-3-yl)methylene]-1,3-dihydro-9H-furo[3,4-b]chromen-9-one-type fluorophores with blue fluorescence emission properties. Tetrahedron 67(10):1837–1843. https://doi.org/10.1016/j.tet.2011.01.033
Teimouri MB, Asnaashari B (2014) One-pot synthesis of 5-(furo[3,4-b]chromenyl)-5-hydroxybarbiturates via a three-component cascade reaction. Tetrahedron Lett 55(14):2249–2252. https://doi.org/10.1016/j.tetlet.2014.02.075
Terzidis MA, Tsiaras VG, Drosos NM, Kasapidou PM, Stephanidou-Stephanatou J, Tsoleridis CA, Buth G, Kostakis GE (2014) Chromeno[2,3-c]pyrroles by one-pot multicomponent domino addition-amination reaction. Tetrahedron Lett 55(41):5601–5604. https://doi.org/10.1016/j.tetlet.2014.08.048
Cioc RC, Ruijter E, Orru RVA (2014) Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem 16(6):2958–2975. https://doi.org/10.1039/C4GC00013G
Tashrifi Z, Mohammadi-Khanaposhtani M, Hamedifar H, Larijani B, Ansari S, Mahdavi M (2020) Synthesis and pharmacological properties of polysubstituted 2-amino-4H-pyran-3-carbonitrile derivatives. Mol Divers 24:1385–1431. https://doi.org/10.1007/s11030-019-09994-9
Brahmbhatt GC, Sutariya TR, Atara HD, Parmar NJ, Gupta VK, Lagunes I, Padrón JM, Murumkar PR, Yadav MR (2020) New pyrazolyl-dibenzo[b, e][1,4]diazepinones: room temperature one-pot synthesis and biological evaluation. Mol Divers 24:355–377. https://doi.org/10.1007/s11030-019-09958-z
Wender PA (2014) Toward the ideal synthesis and molecular function through synthesis-informed design. Nat Prod Rep 31(4):433–440. https://doi.org/10.1039/C4NP00013G
Frasinyuk MS, Khilya VP (1999) Preparation and reactions of isoflavone heteroanalogs (a review). Chem Heterocycl Compd 35:3–22. https://doi.org/10.1007/BF02251655
Ibrahim MA, Ali TE, Alnamer YA, Gabr YA (2010) Synthesis and chemical reactivity of 2-methylchromones. ARKIVOC. https://doi.org/10.3998/ark.5550190.0011.103
Sosnovskikh VY (2003) Synthesis and reactions of halogen-containing chromones Russ. Chem Rev 72(6):489–516. https://doi.org/10.1070/RC2003v072n06ABEH000770
Plaskon AS, Grygorenko OO, Ryabukhin SV (2012) Recyclizations of 3-formylchromones with binucleophiles. Tetrahedron 68(13):2743–2757. https://doi.org/10.1016/j.tet.2012.01.077
Ibrahim MA, El-Kazak AM (2019) Ring opening and recyclization reactions with chromone-3-carbonitrile. J Heterocycl Chem 56(3):1075–1085. https://doi.org/10.1002/jhet.3495
Acknowledgements
Authors are grateful grateful to ENAMINE Ltd., Kyiv for financial support and for providing NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vydzhak, R.N., Panchishin, S.Y., Kachaeva, M.V. et al. Rapid synthetic approaches to libraries of diversified 1,2-dihydrochromeno[2,3-c]pyrrole-3,9-diones and 3-(2-hydroxyphenyl)-4,5-dihydropyrrolo[3,4-c]pyrazol-6(1H)-ones. Mol Divers 26, 1115–1128 (2022). https://doi.org/10.1007/s11030-021-10234-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-021-10234-2