Abstract
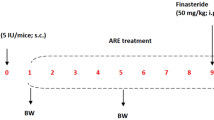
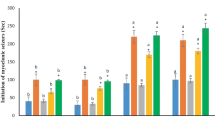
Approximately 40% of women with epilepsy experience perimenstrual seizure exacerbation, referred to as catamenial epilepsy. These seizures result from cyclic changes in circulating progesterone and estradiol levels and there is no effective treatment for this form of intractable epilepsy. We artificially increased progesterone levels and neurosteroid levels (pseudo-pregnancy) in adult Swiss albino female mice (19–23 g) by injecting them with pregnant mares’ serum gonadotropin (5 IU s.c.), followed by human chorionic gonadotropin (5 IU s.c.) after 46 h. After this, ferulic acid (25, 50, 100 mg/kg i.p.) treatment was given for 10 days. During treatment, progesterone, estradiol, and corticosterone levels were estimated in blood on days 1, 5, and 10. Neurosteroid withdrawal was induced by finasteride (50 mg/kg, i.p.) on treatment day 9. Twenty-four hours after finasteride administration (day 10 of treatment), seizure susceptibility was evaluated with the sub-convulsant pentylenetetrazol (PTZ) dose (40 mg/kg i.p.). Four to six hours after PTZ, animals were assessed for depression like phenotypes using tail-suspension test (TST). Four to six hours following TST, animals were euthanized, and discrete brain parts (cortex and hippocampus) were separated for estimation of norepinephrine, serotonin, and dopamine as well as glutamic acid decarboxylase (GAD) enzyme activity. PMSG and HCG treatment elevated progesterone and estradiol levels, assessed on days 1, 5, and 10 causing a state of pseudo-pregnancy. Treatment with finasteride increased seizure susceptibility and depression-like characteristics possibly due to decreased progesterone and elevated estrogen levels coupled with decreased monoamine and elevated corticosterone levels. Ferulic acid treatment, on the other hand, significantly decreased seizure susceptibility and depression like behavior, possibly because of increased progesterone, restored estradiol, corticosterone, monoamines, and GAD enzyme activity. We concluded anticonvulsant effect of ferulic acid in a mouse model of catamenial epilepsy, evidenced by favourable seizure attenuation and curative effect on the circulating progesterone, estradiol, and corticosterone levels along with restorative effect on GAD enzyme activity and monoamine levels.





Similar content being viewed by others
Data Availability
Data will be available on reasonable request.
Code Availability
Not applicable.
References
Andrade S, Silveira SL, Arbo BD et al (2010) Sex-dependent antidepressant effects of lower doses of progesterone in rats. Physiol Behav 99:687–690
Bandopadhyay R, Singh T, Ghoneim MM et al (2021) Recent Developments in Diagnosis of Epilepsy: Scope of MicroRNA and Technological Advancements. Biology 10:1097
Basu T, Maguire J, Salpekar JA (2021) Hypothalamic-pituitary-adrenal axis targets for the treatment of epilepsy.Neurosci Lett135618
Baykara B, Aksu I, Buyuk E et al (2013) Progesterone treatment decreases traumatic brain injury induced anxiety and is correlated with increased serum IGF-1 levels; prefrontal cortex, amygdala, hippocampus neuron density; and reduced serum corticosterone levels in immature rats. Biotech Histochem 88:250–257
Bhatia S, Rawal R, Sharma P et al (2022) Mitochondrial dysfunction in Alzheimer’s disease: Opportunities for drug development. Curr Neuropharmacol 20:675–692
Brodie MJ, Mintzer S, Pack AM et al (2013) Enzyme induction with antiepileptic drugs: Cause for concern? Epilepsia 54; 11–27
Delgado PL (2000) Depression: the case for a monoamine deficiency. J Clin Psychiatry 61:7–11
Guille C, Spencer S, Cavus I et al (2008) The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav 13:12–24
Herzog AG, Fowler KM, Smithson SD et al (2012) Progesterone vs placebo therapy for women with epilepsy: A randomized clinical trial. Neurology 78:1959–1966
Hooper A, Paracha R, Maguire J (2018) Seizure-induced activation of the HPA axis increases seizure frequency and comorbid depression-like behaviors. Epilepsy Behav 78:124–133
Ising M, Künzel HE, Binder EB et al (2005) The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuro-Psycho Biol Psychiatry 29(6):1085–1093
Isojarvi JI, Tauboll E, Herzog AG (2005) Effect of antiepileptic drugs on reproductive endocrine function in individuals with epilepsy. CNS Drug 19:207–223
Joshi S, Kapur J (2019) Neurosteroid regulation of GABAA receptors: a role in catamenial epilepsy. Brain Res 1703:31–40
Karst H, De Kloet ER, Joëls M (1999) Episodic corticosterone treatment accelerates kindling epileptogenesis and triggers long-term changes in hippocampal CA1 cells, in the fully kindled state. Eur J Neuroscience 11:889–898
Kaur N, Singh T, Kumar S, Goel RK (2017) Neurochemical evidence based suggested therapy for safe management of epileptogenesis. Epilepsy Behav 72:8–16
Keshr G, Lakshmi V, Singh MM et al (1999) Post-coital antifertility activity of Ferula asafoetida extract in female rats. Pharmac Biol 37:273–278
Kling MA, Smith MA, Glowa JR et al (1993) Facilitation of cocaine kindling by glucocorticoids in rats. Brain Res 629:163–166
Li Y, Pehrson AL, Budac DP et al (2012) A rodent model of premenstrual dysphoria: progesterone withdrawal induces depression-like behavior that is differentially sensitive to classes of antidepressants. Behav Brain Res 234:238–247
Li Y, Raaby KF, Sánchez C, Gulinello M (2013) Serotonergic receptor mechanisms underlying antidepressant-like action in the progesterone withdrawal model of hormonally induced depression in rats. Behav Brain Res 256:520–528
Li X, Shi G (2021) Therapeutic effects and mechanism of ferulic acid and icariin in mammary gland hyperplasia model rats via regulation of the ERK pathway. Ann Transl Med 9:666
Lloyd KG, Bossi L, Morselli PL et al (1986) Alterations of GABA-mediated synaptic transmission in human epilepsy. Adv Neurol 44:1033–1044
Logothetis J, Harner R, Morrel F (1959) The role of estrogens in catamenial exacerbation of epilepsy. Neurology 9:352–360
Maguire MJ, Nevitt SJ (2019) Treatments for seizures in catamenial (menstrual-related) epilepsy. Cochrane Database Sys Rev 10:CD013225
Mahendra P, Bisht S (2012) Ferula asafoetida: Traditional uses and pharmacological activity. Pharmacogn Rev 6:141
Mishra A, Goel RK (2013) Psychoneurochemical Investigations to Reveal Neurobiology of Memory Deficit in Epilepsy. Neurochem Res 38:2503–2515
Morilak DA, Frazer A (2004) Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol 7:193–218
Pahwa P, Singh T, Goel RK (2022) Anticonvulsant Effect of Asparagus racemosus Willd. In a Mouse Model of Catamenial Epilepsy. Neurochem Res 47:422–433
Patel MV, Hopkins DC, Barr FD et al (2021) Sex Hormones and Aging Modulate Interferon Lambda 1 Production and Signaling by Human Uterine Epithelial Cells and Fibroblasts. Frontiers in Immunology. 12.
Reddy DS (2004) Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3alpha-androstanediol and 17beta-estradiol. Neuroscience 129:195–207
Reddy DS (2009) The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res 85:1–30
Reddy DS (2013) Role of hormones and neurosteroids in epileptogenesis. Front Cell Neurosci 7:115
Reddy DS, Kim HY, Rogawski MA (2001) Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia 42:328–336
Reddy DS, Rogawski MA (2001) Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42:303–310
Roberts AJ, Keith LD (1994) Sensitivity of the circadian rhythm of kainic acid-induced convulsion susceptibility to manipulations of corticosterone levels and mineralocorticoid receptor binding. Neuropharmacology 33:1087–1093
Salih NF, Jaafar MS (2013) Alpha deposit into blood: A new method to evaluate infertility of women by measuring the level of LH, FSH and HCG. J Infertil Reprod Biol 1:21–25
Sharma RK, Singh T, Mishra A et al (2017) Relative safety of different antidepressants for treatment of depression in chronic epileptic animals associated with depression. J Epilepsy Res 7:25
Shiono S, Williamson J, Kapur J et al (2019) Progesterone receptor activation regulates seizure susceptibility. Ann Clin Transl Neurol 6:1302–1310
Singh D, Gawande DY, Singh T et al (2014) Revealing pharmacodynamics of medicinal plants using in silico approach: a case study with wet lab validation. Comput Biol Med 47:1–6
Singh T, Kaur T, Goel RK (2017a) Adjuvant quercetin therapy for combined treatment of epilepsy and comorbid depression. Neurochem Int 104:27–33
Singh T, Bagga N, Kaur A et al (2017b) Agmatine for combined treatment of epilepsy, depression and cognitive impairment in chronic epileptic animals. Biomed Pharmacother 92:720–725
Singh T, Kaur T, Goel RK (2017c) Ferulic acid supplementation for management of depression in epilepsy. Neurochem Res 42:2940–2948
Singh T, Goel RK (2015) Neuroprotective effect of Allium cepa L. in aluminium chloride induced neurotoxicity. Neurotoxicology 49:1–7
Singh T, Goel RK (2016a) Evidence in support of using a neurochemistry approach to identify therapy for both epilepsy and associated depression. Epilepsy Behav 61:248–257
Singh T, Goel RK (2016b) Adjuvant indoleamine 2, 3-dioxygenase enzyme inhibition for comprehensive management of epilepsy and comorbid depression. Eur J Pharmacol 784:111–120
Singh T, Goel RK (2017a) Adjuvant neuronal nitric oxide synthase inhibition for combined treatment of epilepsy and comorbid depression. Pharmacol Rep 69:143–149
Singh T, Goel RK (2017b) Managing epilepsy-associated depression: Serotonin enhancers or serotonin producers? Epilepsy Behav 66:93–99
Singh T, Goel RK (2021) Epilepsy associated depression: An update on current scenario, suggested mechanisms, and opportunities. Neurochem Res 46:1305–1321
Singh T, Joshi S, Williamson JM et al (2020) Neocortical injury–induced status epilepticus. Epilepsia 61:2811–2824
Singh T, Mishra A, Goel RK (2021) PTZ kindling model for epileptogenesis, refractory epilepsy, and associated comorbidities: relevance and reliability. Metab Brain Dis 36:1573–1590
Singh T, Batabyal T, Kapur J (2022a) Neuronal circuits sustaining neocortical-injury-induced status epilepticus. Neurobiol Dis 165:105633
Singh H, Singh T, Singh AP et al (2022b) Hepatoprotective effect of Physalis divaricata in paracetamol induced hepatotoxicity: In vitro, in silico and in vivo analysis. J Ethnopharmacol 290:115024
Singh T, Thapliyal S, Bhatia S et al (2022c) Reconnoitering the transformative journey of minocycline from an antibiotic to an antiepileptic drug. Life Sci 293:120346
Thapliyal S, Singh T, Handu S et al (2021) A Review on Potential Footprints of Ferulic Acid for Treatment of Neurological Disorders. Neurochem Res 46:1043–1057
Velíšková J (2006) The role of estrogens in seizures and epilepsy: the bad guys or the good guys? Neuroscience 138:837–844
Velíšková J (2007) Estrogens and epilepsy: why are we so excited? Neuroscientist 13:77–88
Wallis CJ, Luttge WG (1980) Influence of estrogen and progesterone on glutamic acid decarboxylase activity in discrete regions of rat brain. J Neurochem 34:609–613
Wei JQ, Wei JL, Zhou XT et al (1990) Isocratic reversed phase high performance liquid chromatography determination of twelve natural corticosteroids in serum with on-line ultraviolet and fluorescence detection. Biomed Chromatogr 4:161–164
Williamson J, Singh T, Kapur J (2019) Neurobiology of organophosphate-induced seizures. Epilepsy Behav 101:106426
Wolf R, Klemisch H (1991) Adaptation of an enzymatic fluorescence assay for L-glutamic acid decarboxylase. Anal Biochem 192:78–81
Zia-Ul-Haq M, Shahid SA, Ahmad S, Qayum M, Khan I (2012) Antioxidant potential of various parts of Ferula assafoetida L. J Med Plants Res 6:3254–3258
Funding
The authors are deeply grateful to the Council of Scientific and Industrial Research (CSIR), Pusa, New Delhi, India for providing financial assistance (Vide F. No. 38 (1339)/12/EMR-II) for the project to Dr. Rajesh Kumar Goel.
Author information
Authors and Affiliations
Contributions
HKD and TS performed experiments. HKD wrote the first draft of manuscript. RKG conceived the idea, edited, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
None.
Ethical approval
The animal experiments were obtained the approval from the Animal Ethics Committee of Punjabi University, India.
Consent for publication
All authors have their consents for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Figure. 1
Effect of different treatments on corticosterone, progesterone, and estradiol on day 1, and 5. Each value is expressed as mean ± standard error mean (n = 6). The significance level was considered at P < 0.05 (Student Newman Keuls Post hoc test). *: significant as compared to pseudo pregnant control; # significant as compared to finasteride control; Ω: significant as compared to naïve. Abbreviations: Naïve: saline treatment only; Pseudo: Pseudopregnant control; Fina: Finasteride control; FA 25, 50 and 100: Ferulic acid 25, 50 and 100 mg/kg; i.p.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhillon, H.K., Singh, T. & Goel, R.K. Ferulic acid inhibits catamenial epilepsy through modulation of female hormones. Metab Brain Dis 37, 2827–2838 (2022). https://doi.org/10.1007/s11011-022-01054-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01054-w