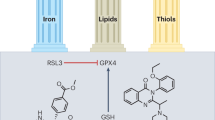
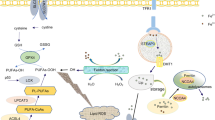
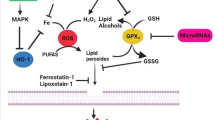
Abstract
Ferroptosis is a newly discovered type of regulated cell death participated in multiple diseases. Different from other classical cell death programs such as necrosis and apoptosis, ferroptosis involving iron-catalyzed lipid peroxidation is characterized by Fe2+ accumulation and mitochondria alterations. The phenomenon of oxidative stress following organ ischemia–reperfusion (I/R) has recently garnered attention for its connection to the onset of ferroptosis and subsequent reperfusion injuries. This article provides a comprehensive overview underlying the mechanisms of ferroptosis, with a further focus on the latest research progress regarding interference with ferroptotic pathways in organ I/R injuries, such as intestine, lung, heart, kidney, liver, and brain. Understanding the links between ferroptosis and I/R injury may inform potential therapeutic strategies and targeted agents.








Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- I/R:
-
Ischemia–reperfusion
- IRI:
-
Ischemia–reperfusion injury
- OIRIs:
-
Organ ischemia–reperfusion injuries
- RCD:
-
Regulated cell death
- ROS:
-
Reactive oxygen species
- PUFA:
-
Polyunsaturated fatty acid
- LOX:
-
Lipoxygenase
- Lipid-OOH:
-
Lipid peroxides
- TF:
-
Transferrin
- TfR1:
-
Transferrin receptor protein-1
- STEAP3:
-
Prostate six-transmembrane epithelial antigen 3
- DMT1:
-
Divalent metal transporter 1
- LIP:
-
Labile iron pool
- PCBP1:
-
Poly-(rC)-binding protein 1
- PCBP2:
-
Poly-(rC)-binding protein 2
- FPN1:
-
Ferroportin
- SLC7A11:
-
Solute carrier family 7 member 11
- xCT:
-
Cysteine/glutamate transporter
- SLC3A2:
-
Solute carrier family 3 member 2
- 4F2hc:
-
4F2 heavy chain
- GSH:
-
Glutathione
- •OH:
-
Hydroxyl radicals
- O2• − :
-
Superoxide anions
- H2O2:
-
Hydrogen peroxide
- GPX:
-
Glutathione peroxidases
- Lipid-OH:
-
Lipid alcohol
- GSSG:
-
Glutathione disulfide
- GR:
-
Glutathione reductase
- NADPH:
-
Nicotinamide-adenine dinucleotide phosphate
- FA:
-
Fatty acid
- PL:
-
Phospholipid
- SFA:
-
Saturated fatty acid
- MUFA:
-
Monounsaturated fatty acid
- PUFA:
-
Polyunsaturated fatty acid
- ACSL4:
-
Acyl-CoA synthetase long-chain family member-4
- LPCAT3:
-
Lysophosphatidylcholine acyltransferase-3
- PE:
-
Phosphatidylethanolamine
- AA:
-
Arachidonic acid
- AdA:
-
Adrenic acid
- MDA:
-
Malondialdehyde
- 4-HNE:
-
4-Hydroxy-2-nonenal
- IIRI:
-
Intestinal ischemia–reperfusion injury
- CAT:
-
Capsiate
- TRPV1:
-
Transient receptor potential cation channel subfamily V member 1
- H/R:
-
Hypoxia-reoxygenation
- Sp1:
-
Special protein 1
- APG:
-
Apigenin-7-O-β-D-(-6″-p-coumaroyl)-glucopyranoside
- HO-1:
-
Heme oxygenase 1
- MAO-B:
-
Monoamine oxidase b
- ALI:
-
Acute lung injury
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- ARE:
-
Antioxidant reaction element
- Nrf2−/− :
-
Nuclear factor erythroid 2-related factor 2 gene knockout
- TERT:
-
Telomerase reverse transcriptase
- STAT3:
-
Signal transducer and activator of transcription 3
- BCAA:
-
Branched-chain amino acids
- L-BAIBA:
-
L-β-aminoisobutyric acid
- LIRI:
-
Lung ischemia–reperfusion injury
- MIRI:
-
Myocardial ischemia–reperfusion injury
- USP7:
-
Ubiquitin-specific protease 7
- FTH1:
-
Ferritin heavy chain 1
- ALOX15:
-
Arachidonate 15-lipoxygenase
- 15-HpETE:
-
15-Hydroperoxyeicosatetraenoic acid
- Pgc1α:
-
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- RNF34:
-
Ubiquitin ligase ring finger protein 34
- KMT2B:
-
Lysine-specific methyltransferase 2B
- RFK:
-
Riboflavin kinase
- TNF:
-
Tumor necrosis factor
- NOX2:
-
Nicotinamide-adenine dinucleotide phosphate oxidase 2
- ATF3:
-
Activating transcription factor 3
- FANCD2:
-
Fanconi anemia complementation group D2
- RIRI:
-
Renal ischemia–reperfusion injury
- AKI:
-
Acute kidney injury
- Dex:
-
Dexmedetomidine
- α2-AR:
-
α2-Adrenergic receptor
- OGD/R:
-
Oxygen–glucose deprivation and reoxygenation
- USC:
-
Urine-derived stem cell
- USC-Exo:
-
Urine-derived stem cell-derived exosome
- TUG1:
-
Taurine-upregulated gene 1
- SRSF1:
-
Serine/arginine splicing factor 1
- IRE1:
-
Inositol requiring enzyme 1
- JNK:
-
C-Jun NH2-terminal kinases
- CCL2:
-
Chemokine (C–C motif) ligand 2
- CCL7:
-
Chemokine (C–C motif) ligand 7
- HIRI:
-
Hepatic ischemia–reperfusion injury
- LT:
-
Liver transplantation
- Ptgs2:
-
Prostaglandin-endoperoxide synthase 2
- TMEM16A:
-
Transmembrane member 16A
- MSC-Exo:
-
Mesenchymal stem cell-derived exosome
- miR:
-
MicroRNA
- BMMSC:
-
Bone marrow mesenchymal stem cell
- IREB2:
-
Iron response element-binding protein 2
- MCTR1:
-
Maresin conjugate 1 in tissue regeneration
- DMF:
-
Dimethyl fumarate
- ME1:
-
Malic enzyme 1
- PTEN:
-
Phosphatase and tensin homolog
- mTOR:
-
Mechanistic target of rapamycin
- SREBP1:
-
Sterol regulatory element-binding protein 1
- IS:
-
Ischemic stroke
- CIRI:
-
Cerebral ischemia–reperfusion injury
- NQO1:
-
Quinone oxidoreductase 1
- MCAO/R:
-
Middle cerebral artery occlusion and reperfusion
- ELA:
-
Elabela
- APJ:
-
Apelin receptor
- NLRP3:
-
Nucleotide-binding oligomerization domain-like receptor pyrin domain-containing 3
- FACL4:
-
Fatty acid-CoA ligase 4
- PGE2:
-
Prostaglandin E2
- COX:
-
Cyclooxygenase
- NCOA4:
-
Nuclear receptor coactivator 4
- SOD:
-
Superoxide dismutase
- LDH:
-
Lactate dehydrogenase
- LPO:
-
Lipid peroxidase
- HETE:
-
Hydroxyeicosatetraenoic acid
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- HNF4:
-
Hepatocyte nuclear factor-4
References
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379. https://doi.org/10.1038/cdd.2015.158
Wang H, Liu C, Zhao Y, Gao G (2020) Mitochondria regulation in ferroptosis. Eur J Cell Biol 99:151058. https://doi.org/10.1016/j.ejcb.2019.151058
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G (2020) Ferroptosis: past, present and future. Cell Death Dis 11:88. https://doi.org/10.1038/s41419-020-2298-2
Lei P, Bai T, Sun Y (2019) Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol 10:139. https://doi.org/10.3389/fphys.2019.00139
Chen X, Li J, Kang R, Klionsky DJ, Tang D (2021) Ferroptosis: machinery and regulation. Autophagy 17:2054–2081. https://doi.org/10.1080/15548627.2020.1810918
Lee JY, Kim WK, Bae KH, Lee SC, Lee EW (2021) Lipid metabolism and ferroptosis. Biology (Basel). https://doi.org/10.3390/biology10030184
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion-from mechanism to translation. Nat Med 17:1391–1401. https://doi.org/10.1038/nm.2507
Pan Y, Wang X, Liu X, Shen L, Chen Q, Shu Q (2022) Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants (Basel). https://doi.org/10.3390/antiox11112196
Zhou L, Han S, Guo J, Qiu T, Zhou J, Shen L (2022) Ferroptosis-a new dawn in the treatment of organ ischemia-reperfusion injury. Cells. https://doi.org/10.3390/cells11223653
Chen DQ, Guo Y, Li X, Zhang GQ, Li P (2022) Small molecules as modulators of regulated cell death against ischemia/reperfusion injury. Med Res Rev 42:2067–2101. https://doi.org/10.1002/med.21917
Milto IV, Suhodolo IV, Prokopieva VD, Klimenteva TK (2016) Molecular and cellular bases of iron metabolism in humans. Biochemistry (Mosc) 81:549–564. https://doi.org/10.1134/s0006297916060018
Stoyanovsky DA, Tyurina YY, Shrivastava I, Bahar I, Tyurin VA, Protchenko O, Jadhav S, Bolevich SB, Kozlov AV, Vladimirov YA, Shvedova AA, Philpott CC, Bayir H, Kagan VE (2019) Iron catalysis of lipid peroxidation in ferroptosis: regulated enzymatic or random free radical reaction? Free Radic Biol Med 133:153–161. https://doi.org/10.1016/j.freeradbiomed.2018.09.008
Frazer DM, Anderson GJ (2014) The regulation of iron transport. BioFactors 40:206–214. https://doi.org/10.1002/biof.1148
Lane DJ, Merlot AM, Huang ML, Bae DH, Jansson PJ, Sahni S, Kalinowski DS, Richardson DR (2015) Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim Biophys Acta 1853:1130–1144. https://doi.org/10.1016/j.bbamcr.2015.01.021
Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y (2016) Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci 41:274–286. https://doi.org/10.1016/j.tibs.2015.11.012
Vogt AS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF (2021) On iron metabolism and its regulation. Int J Mol Sci. https://doi.org/10.3390/ijms22094591
Lewerenz J, Hewett SJ, Huang Y, Lambros M, Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M, Smith SB, Ganapathy V, Maher P (2013) The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18:522–555. https://doi.org/10.1089/ars.2011.4391
Conrad M, Sato H (2012) The oxidative stress-inducible cystine/glutamate antiporter, system x (c) (-): cystine supplier and beyond. Amino Acids 42:231–246. https://doi.org/10.1007/s00726-011-0867-5
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. https://doi.org/10.1016/j.bbagen.2012.09.008
Xu C, Liu Z, Xiao J (2021) Ferroptosis: a double-edged sword in gastrointestinal disease. Int J Mol Sci. https://doi.org/10.3390/ijms222212403
Feng Q, Yu X, Qiao Y, Pan S, Wang R, Zheng B, Wang H, Ren KD, Liu H, Yang Y (2022) Ferroptosis and acute kidney injury (AKI): molecular mechanisms and therapeutic potentials. Front Pharmacol 13:858676. https://doi.org/10.3389/fphar.2022.858676
Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z, Lin H, Fang X, Qu Y, Wang Y, Shen Z, Zhao M, Cui X (2021) Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev 2021:6614009. https://doi.org/10.1155/2021/6614009
Kajarabille N, Latunde-Dada GO (2019) Programmed cell-death by ferroptosis: antioxidants as mitigators. Int J Mol Sci. https://doi.org/10.3390/ijms20194968
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44:532–553. https://doi.org/10.1159/000485089
Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan X, Wu C (2021) Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 277:121110. https://doi.org/10.1016/j.biomaterials.2021.121110
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012:736837. https://doi.org/10.1155/2012/736837
Liu T, Sun L, Zhang Y, Wang Y, Zheng J (2022) Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol 36:e22942. https://doi.org/10.1002/jbt.22942
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 152:175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduct Target Ther 6:49. https://doi.org/10.1038/s41392-020-00428-9
Elinder F, Liin SI (2017) Actions and mechanisms of polyunsaturated fatty acids on voltage-gated ion channels. Front Physiol 8:43. https://doi.org/10.3389/fphys.2017.00043
Conrad M, Pratt DA (2019) The chemical basis of ferroptosis. Nat Chem Biol 15:1137–1147. https://doi.org/10.1038/s41589-019-0408-1
Xu S, He Y, Lin L, Chen P, Chen M, Zhang S (2021) The emerging role of ferroptosis in intestinal disease. Cell Death Dis 12:289. https://doi.org/10.1038/s41419-021-03559-1
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13:91–98. https://doi.org/10.1038/nchembio.2239
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, Xu S, Gao Y, Chen X, Sui X, Li G (2020) The emerging role of ferroptosis in inflammation. Biomed Pharmacother 127:110108. https://doi.org/10.1016/j.biopha.2020.110108
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113:E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayır H (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13:81–90. https://doi.org/10.1038/nchembio.2238
Haeggström JZ, Funk CD (2011) Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem Rev 111:5866–5898. https://doi.org/10.1021/cr200246d
Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35:830–849. https://doi.org/10.1016/j.ccell.2019.04.002
Mallick IH, Yang W, Winslet MC, Seifalian AM (2004) Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci 49:1359–1377. https://doi.org/10.1023/b:ddas.0000042232.98927.91
Gonzalez LM, Moeser AJ, Blikslager AT (2015) Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am J Physiol Gastrointest Liver Physiol 308:G63-75. https://doi.org/10.1152/ajpgi.00112.2013
Akbari G (2020) Emerging roles of microRNAs in intestinal ischemia/reperfusion-induced injury: a review. J Physiol Biochem 76:525–537. https://doi.org/10.1007/s13105-020-00772-y
Deng F, Zhao BC, Yang X, Lin ZB, Sun QS, Wang YF, Yan ZZ, Liu WF, Li C, Hu JJ, Liu KX (2021) The gut microbiota metabolite capsiate promotes Gpx4 expression by activating TRPV1 to inhibit intestinal ischemia reperfusion-induced ferroptosis. Gut Microbes 13:1–21. https://doi.org/10.1080/19490976.2021.1902719
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, Tian X (2019) Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 26:2284–2299. https://doi.org/10.1038/s41418-019-0299-4
Feng YD, Ye W, Tian W, Meng JR, Zhang M, Sun Y, Zhang HN, Wang SJ, Wu KH, Liu CX, Liu SY, Cao W, Li XQ (2022) Old targets, new strategy: apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside prevents endothelial ferroptosis and alleviates intestinal ischemia-reperfusion injury through HO-1 and MAO-B inhibition. Free Radic Biol Med 184:74–88. https://doi.org/10.1016/j.freeradbiomed.2022.03.033
Zhang F, Li ZL, Xu XM, Hu Y, Yao JH, Xu W, Jing HR, Wang S, Ning SL, Tian XF (2015) Protective effects of icariin-mediated SIRT1/FOXO3 signaling pathway on intestinal ischemia/reperfusion-induced acute lung injury. Mol Med Rep 11:269–276. https://doi.org/10.3892/mmr.2014.2679
Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J (2016) Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 73:3221–3247. https://doi.org/10.1007/s00018-016-2223-0
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107. https://doi.org/10.1016/j.redox.2019.101107
Dong H, Xia Y, Jin S, Xue C, Wang Y, Hu R, Jiang H (2021) Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis 12:1027. https://doi.org/10.1038/s41419-021-04307-1
Dong H, Qiang Z, Chai D, Peng J, Xia Y, Hu R, Jiang H (2020) Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging (Albany NY) 12:12943–12959. https://doi.org/10.18632/aging.103378
Qiang Z, Dong H, Xia Y, Chai D, Hu R, Jiang H (2020) Nrf2 and STAT3 alleviates ferroptosis-mediated IIR-ALI by regulating SLC7A11. Oxid Med Cell Longev 2020:5146982. https://doi.org/10.1155/2020/5146982
Hochegger K, Koppelstaetter C, Tagwerker A, Huber JM, Heininger D, Mayer G, Rosenkranz AR (2007) p21 and mTERT are novel markers for determining different ischemic time periods in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 292:F762–F768. https://doi.org/10.1152/ajprenal.00084.2006
Indran IR, Hande MP, Pervaiz S (2011) hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res 71:266–276. https://doi.org/10.1158/0008-5472.Can-10-1588
Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, Liao P, Zhou J, Zhang Q, Dow A, Saripalli AL, Kryczek I, Wei S, Szeliga W, Vatan L, Stone EM, Georgiou G, Cieslik M, Wahl DR, Morgan MA, Chinnaiyan AM, Lawrence TS, Zou W (2019) Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov 9:1673–1685. https://doi.org/10.1158/2159-8290.Cd-19-0338
Xu Y, Li X, Cheng Y, Yang M, Wang R (2020) Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J 34:16262–16275. https://doi.org/10.1096/fj.202001758R
Talaie T, DiChiacchio L, Prasad NK, Pasrija C, Julliard W, Kaczorowski DJ, Zhao Y, Lau CL (2021) Ischemia-reperfusion injury in the transplanted lung: a literature review. Trans Direct 7:e652. https://doi.org/10.1097/txd.0000000000001104
Lian K, Guo X, Wang Q, Liu Y, Wang RT, Gao C, Li CY, Li CX, Tao L (2020) PP2Cm overexpression alleviates MI/R injury mediated by a BCAA catabolism defect and oxidative stress in diabetic mice. Eur J Pharmacol 866:172796. https://doi.org/10.1016/j.ejphar.2019.172796
Zhang Z, Li X, Guo J, He B, Wu L, Yang R, Li X, Fang D, Yang X, Yang D, Wang F, Tang M, Han Y, Jose PA, Wang H, Zeng C (2023) β-Aminoisobutyrics acid, a metabolite of BCAA, activates the AMPK/Nrf-2 pathway to prevent ferroptosis and ameliorates lung ischemia-reperfusion injury. Mol Med 29:164. https://doi.org/10.1186/s10020-023-00729-z
Shanmugasundaram D, Fan Q, Wang M, Yi R, Wang O (2022) Safety assessment of L-β-aminoisobutyric acid (L-BAIBA): subchronic toxicity study in Sprague Dawley rats. Int J Toxicol 41:329–346. https://doi.org/10.1177/10915818221094487
Lyssikatos C, Wang Z, Liu Z, Warden SJ, Brotto M, Bonewald L (2023) L-β-Aminoisobutyric acid, L-BAIBA, a marker of bone mineral density and body mass index, and D-BAIBA of physical performance and age. Sci Rep 13:17212. https://doi.org/10.1038/s41598-023-44249-6
Wang Y, Chen Z, Luo J, Zhang J, Sang AM, Cheng ZS, Li XY (2023) Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. Int Immunopharmacol 115:109731. https://doi.org/10.1016/j.intimp.2023.109731
Ryter SW (2022) Heme oxygenase-1: an anti-inflammatory effector in cardiovascular, lung, and related metabolic disorders. Antioxidants (Basel). https://doi.org/10.3390/antiox11030555
Consoli V, Sorrenti V, Grosso S, Vanella L (2021) Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules. https://doi.org/10.3390/biom11040589
Kikuchi G, Yoshida T, Noguchi M (2005) Heme oxygenase and heme degradation. Biochem Biophys Res Commun 338:558–567. https://doi.org/10.1016/j.bbrc.2005.08.020
Chiabrando D, Vinchi F, Fiorito V, Mercurio S, Tolosano E (2014) Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol 5:61. https://doi.org/10.3389/fphar.2014.00061
Ryter SW, Tyrrell RM (2000) The heme synthesis and degradation pathways: role in oxidant sensitivity. heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28:289–309. https://doi.org/10.1016/s0891-5849(99)00223-3
Zhang X, Ding M, Zhu P, Huang H, Zhuang Q, Shen J, Cai Y, Zhao M, He Q (2019) New insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxid Med Cell Longev 2019:3214196. https://doi.org/10.1155/2019/3214196
Ryter SW (2021) Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells. https://doi.org/10.3390/cells10030515
Wang Y, Dong Z, Zhang Z, Wang Y, Yang K, Li X (2022) Postconditioning with Irisin attenuates lung ischemia/reperfusion injury by suppressing ferroptosis via induction of the Nrf2/HO-1 signal axis. Oxid Med Cell Longev 2022:9911167. https://doi.org/10.1155/2022/9911167
Zhao J, Li J, Wei D, Gao F, Yang X, Yue B, Xiong D, Liu M, Xu H, Hu C, Chen J (2023) Liproxstatin-1 alleviates lung transplantation-induced cold ischemia-reperfusion injury by inhibiting ferroptosis. Transplantation 107:2190–2202. https://doi.org/10.1097/tp.0000000000004638
Zhou M, Yu Y, Luo X, Wang J, Lan X, Liu P, Feng Y, Jian W (2021) Myocardial ischemia-reperfusion injury: therapeutics from a mitochondria-centric perspective. Cardiology 146:781–792. https://doi.org/10.1159/000518879
He J, Liu D, Zhao L, Zhou D, Rong J, Zhang L, Xia Z (2022) Myocardial ischemia/reperfusion injury: mechanisms of injury and implications for management (review). Exp Ther Med 23:430. https://doi.org/10.3892/etm.2022.11357
Lillo-Moya J, Rojas-Solé C, Muñoz-Salamanca D, Panieri E, Saso L, Rodrigo R (2021) Targeting ferroptosis against ischemia/reperfusion cardiac injury. Antioxidants (Basel). https://doi.org/10.3390/antiox10050667
Zhao WK, Zhou Y, Xu TT, Wu Q (2021) Ferroptosis: opportunities and challenges in myocardial ischemia-reperfusion injury. Oxid Med Cell Longev 2021:9929687. https://doi.org/10.1155/2021/9929687
Candelaria PV, Leoh LS, Penichet ML, Daniels-Wells TR (2021) Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents. Front Immunol 12:607692. https://doi.org/10.3389/fimmu.2021.607692
Feng H, Schorpp K, Jin J, Yozwiak CE, Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka JM, Upadhyayula PS, Canoll P, Uchida K, Soni RK, Hadian K, Stockwell BR (2020) Transferrin receptor is a specific ferroptosis marker. Cell Rep 30:3411-3423.e7. https://doi.org/10.1016/j.celrep.2020.02.049
Tang LJ, Zhou YJ, Xiong XM, Li NS, Zhang JJ, Luo XJ, Peng J (2021) Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic Biol Med 162:339–352. https://doi.org/10.1016/j.freeradbiomed.2020.10.307
Zhang C, Liu J, Wang J, Zhang T, Xu D, Hu W, Feng Z (2021) The interplay between tumor suppressor p53 and hypoxia signaling pathways in cancer. Front Cell Dev Biol 9:648808. https://doi.org/10.3389/fcell.2021.648808
Chibaya L, Karim B, Zhang H, Jones SN (2021) Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.2003193118
Li S, Lei Z, Yang X, Zhao M, Hou Y, Wang D, Tang S, Li J, Yu J (2022) Propofol protects myocardium from ischemia/reperfusion injury by inhibiting ferroptosis through the AKT/p53 signaling pathway. Front Pharmacol 13:841410. https://doi.org/10.3389/fphar.2022.841410
Xuan W, Lu X, Yang Z, Li J, Jin W, Li Y (2022) Propofol protects against erastin-induced ferroptosis in HT-22 cells. J Mol Neurosci 72:1797–1808. https://doi.org/10.1007/s12031-022-02017-7
Snodgrass RG, Brüne B (2019) Regulation and functions of 15-lipoxygenases in human macrophages. Front Pharmacol 10:719. https://doi.org/10.3389/fphar.2019.00719
Çolakoğlu M, Tunçer S, Banerjee S (2018) Emerging cellular functions of the lipid metabolizing enzyme 15-lipoxygenase-1. Cell Prolif 51:e12472. https://doi.org/10.1111/cpr.12472
Ma XH, Liu JH, Liu CY, Sun WY, Duan WJ, Wang G, Kurihara H, He RR, Li YF, Chen Y, Shang H (2022) ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct Target Ther 7:288. https://doi.org/10.1038/s41392-022-01090-z
Cai W, Liu L, Shi X, Liu Y, Wang J, Fang X, Chen Z, Ai D, Zhu Y, Zhang X (2023) Alox15/15-HpETE aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation 147:1444–1460. https://doi.org/10.1161/circulationaha.122.060257
Cao Y, Luo F, Peng J, Fang Z, Liu Q, Zhou S (2022) KMT2B-dependent RFK transcription activates the TNF-α/NOX2 pathway and enhances ferroptosis caused by myocardial ischemia-reperfusion. J Mol Cell Cardiol 173:75–91. https://doi.org/10.1016/j.yjmcc.2022.09.003
Liu H, Mo H, Yang C, Mei X, Song X, Lu W, Xiao H, Yan J, Wang X, Yan J, Luo T, Lin Y, Wen D, Chen G, Chen A, Ling Y (2022) A novel function of ATF3 in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion. Free Radic Biol Med 189:122–135. https://doi.org/10.1016/j.freeradbiomed.2022.07.006
Ye J, Lyu TJ, Li LY, Liu Y, Zhang H, Wang X, Xi X, Liu ZJ, Gao JQ (2023) Ginsenoside Re attenuates myocardial ischemia/reperfusion induced ferroptosis via miR-144-3p/SLC7A11. Phytomedicine 113:154681. https://doi.org/10.1016/j.phymed.2023.154681
Qian W, Liu D, Han Y, Liu M, Liu B, Ji Q, Zhang B, Mei Q, Zhou S, Cheng Y (2023) Cyclosporine A-loaded apoferritin alleviates myocardial ischemia-reperfusion injury by simultaneously blocking ferroptosis and apoptosis of cardiomyocytes. Acta Biomater 160:265–280. https://doi.org/10.1016/j.actbio.2023.02.025
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Kleinbongard P, Amanakis G, Skyschally A, Heusch G (2018) Reflection of cardioprotection by remote ischemic perconditioning in attenuated ST-segment elevation during ongoing coronary occlusion in pigs: evidence for cardioprotection from ischemic injury. Circ Res 122:1102–1108. https://doi.org/10.1161/circresaha.118.312784
Tissier R, Ghaleh B, Cohen MV, Downey JM, Berdeaux A (2012) Myocardial protection with mild hypothermia. Cardiovasc Res 94:217–225. https://doi.org/10.1093/cvr/cvr315
Smith SF, Hosgood SA, Nicholson ML (2019) Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int 95:50–56. https://doi.org/10.1016/j.kint.2018.10.009
Zhao H, Alam A, Soo AP, George AJT, Ma D (2018) Ischemia-reperfusion injury reduces long term renal graft survival: mechanism and beyond. EBioMedicine 28:31–42. https://doi.org/10.1016/j.ebiom.2018.01.025
Han SJ, Lee HT (2019) Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res Clin Pract 38:427–440. https://doi.org/10.23876/j.krcp.19.062
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet 394:1949–1964. https://doi.org/10.1016/s0140-6736(19)32563-2
Bonventre JV, Yang L (2011) Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121:4210–4221. https://doi.org/10.1172/jci45161
Belavgeni A, Meyer C, Stumpf J, Hugo C, Linkermann A (2020) Ferroptosis and necroptosis in the kidney. Cell Chem Biol 27:448–462. https://doi.org/10.1016/j.chembiol.2020.03.016
Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Bräsen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S (2014) Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A 111:16836–16841. https://doi.org/10.1073/pnas.1415518111
Hosohata K, Harnsirikarn T, Chokesuwattanaskul S (2022) Ferroptosis: a potential therapeutic target in acute kidney injury. Int J Mol Sci. https://doi.org/10.3390/ijms23126583
Yuan H, Li X, Zhang X, Kang R, Tang D (2016) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 478:1338–1343. https://doi.org/10.1016/j.bbrc.2016.08.124
Tao WH, Shan XS, Zhang JX, Liu HY, Wang BY, Wei X, Zhang M, Peng K, Ding J, Xu SX, Li LG, Hu JK, Meng XW, Ji FH (2022) Dexmedetomidine attenuates ferroptosis-mediated renal ischemia/reperfusion injury and inflammation by inhibiting ACSL4 via α2-AR. Front Pharmacol 13:782466. https://doi.org/10.3389/fphar.2022.782466
Si Y, Bao H, Han L, Shi H, Zhang Y, Xu L, Liu C, Wang J, Yang X, Vohra A, Ma D (2013) Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med 11:141. https://doi.org/10.1186/1479-5876-11-141
Si Y, Bao H, Han L, Chen L, Zeng L, Jing L, Xing Y, Geng Y (2018) Dexmedetomidine attenuation of renal ischaemia-reperfusion injury requires sirtuin 3 activation. Br J Anaesth 121:1260–1271. https://doi.org/10.1016/j.bja.2018.07.007
Sun Z, Wu J, Bi Q, Wang W (2022) Exosomal lncRNA TUG1 derived from human urine-derived stem cells attenuates renal ischemia/reperfusion injury by interacting with SRSF1 to regulate ASCL4-mediated ferroptosis. Stem Cell Res Ther 13:297. https://doi.org/10.1186/s13287-022-02986-x
Liang Y, Liu Z, Qu L, Wang Y, Zhou Y, Liang L, Guo Y, Tang L (2022) Inhibition of the IRE1/JNK pathway in renal tubular epithelial cells attenuates ferroptosis in acute kidney injury. Front Pharmacol 13:927641. https://doi.org/10.3389/fphar.2022.927641
Arendt J and Aulinas A (2000) Physiology of the Pineal Gland and Melatonin. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Hofland J, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL and Wilson DP (eds) Endotext, MDText.com, Inc. Copyright © 2000–2023, MDText.com, Inc., South Dartmouth (MA)
Huang YB, Jiang L, Liu XQ, Wang X, Gao L, Zeng HX, Zhu W, Hu XR, Wu YG (2022) Melatonin alleviates acute kidney injury by inhibiting NRF2/Slc7a11 axis-mediated ferroptosis. Oxid Med Cell Longev 2022:4776243. https://doi.org/10.1155/2022/4776243
Batiha GE, Beshbishy AM, Ikram M, Mulla ZS, El-Hack MEA, Taha AE, Algammal AM, Elewa YHA (2020) The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin. Foods. https://doi.org/10.3390/foods9030374
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R, Cui X, Yang H, Yang Y, Birnbaumer L, Li X, Gao X (2021) Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res 28:231–243. https://doi.org/10.1016/j.jare.2020.07.007
Chen C, Wang D, Yu Y, Zhao T, Min N, Wu Y, Kang L, Zhao Y, Du L, Zhang M, Gong J, Zhang Z, Zhang Y, Mi X, Yue S, Tan X (2021) Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis 12:65. https://doi.org/10.1038/s41419-020-03362-4
Miller G, Matthews SP, Reinheckel T, Fleming S, Watts C (2011) Asparagine endopeptidase is required for normal kidney physiology and homeostasis. Faseb j 25:1606–1617. https://doi.org/10.1096/fj.10-172312
Dall E, Brandstetter H (2016) Structure and function of legumain in health and disease. Biochimie 122:126–150. https://doi.org/10.1016/j.biochi.2015.09.022
Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW (2013) Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat Rev Gastroenterol Hepatol 10:79–89. https://doi.org/10.1038/nrgastro.2012.225
Liu H, Man K (2021) New insights in mechanisms and therapeutics for short- and long-term impacts of hepatic ischemia reperfusion injury post liver transplantation. Int J Mol Sci. https://doi.org/10.3390/ijms22158210
Yamada N, Karasawa T, Wakiya T, Sadatomo A, Ito H, Kamata R, Watanabe S, Komada T, Kimura H, Sanada Y, Sakuma Y, Mizuta K, Ohno N, Sata N, Takahashi M (2020) Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am J Transplant 20:1606–1618. https://doi.org/10.1111/ajt.15773
Rishi G, Subramaniam VN (2017) The liver in regulation of iron homeostasis. Am J Physiol Gastrointest Liver Physiol 313:G157-g165. https://doi.org/10.1152/ajpgi.00004.2017
Wu S, Yang J, Sun G, Hu J, Zhang Q, Cai J, Yuan D, Li H, Hei Z, Yao W (2021) Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br J Pharmacol 178:3783–3796. https://doi.org/10.1111/bph.15518
Guo J, Song Z, Yu J, Li C, Jin C, Duan W, Liu X, Liu Y, Huang S, Tuo Y, Pei F, Jian Z, Zhou P, Zheng S, Zou Z, Zhang F, Gong Q, Liang S (2022) Hepatocyte-specific TMEM16A deficiency alleviates hepatic ischemia/reperfusion injury via suppressing GPX4-mediated ferroptosis. Cell Death Dis 13:1072. https://doi.org/10.1038/s41419-022-05518-w
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U (2008) TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455:1210–1215. https://doi.org/10.1038/nature07313
Guo JW, Liu X, Zhang TT, Lin XC, Hong Y, Yu J, Wu QY, Zhang FR, Wu QQ, Shang JY, Lv XF, Ou JS, Zhou JG, Pang RP, Tang BD, Liang SJ (2020) Hepatocyte TMEM16A deletion retards NAFLD progression by ameliorating hepatic glucose metabolic disorder. Adv Sci (Weinh) 7:1903657. https://doi.org/10.1002/advs.201903657
Li X, Wu L, Tian X, Zheng W, Yuan M, Tian X, Zuo H, Song H, Shen Z (2022) miR-29a-3p in exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells alleviates steatotic liver ischemia-reperfusion injury in rats by suppressing ferroptosis via iron responsive element binding protein 2. Oxid Med Cell Longev 2022:6520789. https://doi.org/10.1155/2022/6520789
Wu L, Tian X, Zuo H, Zheng W, Li X, Yuan M, Tian X, Song H (2022) miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J Nanobiotechnology 20:196. https://doi.org/10.1186/s12951-022-01407-8
Ye J, Peng J, Liu K, Zhang T, Huang W (2022) MCTR1 inhibits ferroptosis by promoting NRF2 expression to attenuate hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 323:G283-g293. https://doi.org/10.1152/ajpgi.00354.2021
Qi D, Chen P, Bao H, Zhang L, Sun K, Song S, Li T (2023) Dimethyl fumarate protects against hepatic ischemia-reperfusion injury by alleviating ferroptosis via the NRF2/SLC7A11/HO-1 axis. Cell Cycle 22:818–828. https://doi.org/10.1080/15384101.2022.2155016
Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur BW, Petasis NA, Chiang N, Serhan CN (2016) Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc Natl Acad Sci U S A 113:12232–12237. https://doi.org/10.1073/pnas.1607003113
Xiao J, Yang Q, Zhang Y, Xu H, Ye Y, Li L, Yang Y, Jin S (2021) Maresin conjugates in tissue regeneration-1 suppresses ferroptosis in septic acute kidney injury. Cell Biosci 11:221. https://doi.org/10.1186/s13578-021-00734-x
Tastan B, Arioz BI, Tufekci KU, Tarakcioglu E, Gonul CP, Genc K, Genc S (2021) Dimethyl fumarate alleviates NLRP3 inflammasome activation in microglia and sickness behavior in LPS-challenged mice. Front Immunol 12:737065. https://doi.org/10.3389/fimmu.2021.737065
Fang X, Zhang J, Li Y, Song Y, Yu Y, Cai Z, Lian F, Yang J, Min J, Wang F (2023) Malic enzyme 1 as a novel anti-ferroptotic regulator in hepatic ischemia/reperfusion injury. Adv Sci (Weinh). https://doi.org/10.1002/advs.202205436
Pongratz RL, Kibbey RG, Shulman GI, Cline GW (2007) Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem 282:200–207. https://doi.org/10.1074/jbc.M602954200
Simmen FA, Pabona JMP, Al-Dwairi A, Alhallak I, Montales MTE, Simmen RCM (2023) Malic enzyme 1 (ME1) promotes adiposity and hepatic steatosis and induces circulating insulin and leptin in obese female mice. Int J Mol Sci. https://doi.org/10.3390/ijms24076613
Wu Y, Jiao H, Yue Y, He K, Jin Y, Zhang J, Zhang J, Wei Y, Luo H, Hao Z, Zhao X, Xia Q, Zhong Q, Zhang J (2022) Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ 29:1705–1718. https://doi.org/10.1038/s41418-022-00957-6
Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA (2019) Ischaemic stroke. Nat Rev Dis Primers 5:70. https://doi.org/10.1038/s41572-019-0118-8
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang NY, Tsao CW (2021) Heart disease and stroke statistics-2021 update: a report from the american heart association. Circulation 143:e254–e743. https://doi.org/10.1161/cir.0000000000000950
Ospel JM, Holodinsky JK, Goyal M (2020) Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol 75:1832–1843. https://doi.org/10.1016/j.jacc.2019.10.034
Mendelson SJ, Prabhakaran S (2021) Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA 325:1088–1098. https://doi.org/10.1001/jama.2020.26867
Hughes RE, Tadi P and Bollu PC (2023) TPA therapy. StatPearls, StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC., Treasure Island (FL)
George PM, Steinberg GK (2015) Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87:297–309. https://doi.org/10.1016/j.neuron.2015.05.041
Datta A, Sarmah D, Mounica L, Kaur H, Kesharwani R, Verma G, Veeresh P, Kotian V, Kalia K, Borah A, Wang X, Dave KR, Yavagal DR, Bhattacharya P (2020) Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl Stroke Res 11:1185–1202. https://doi.org/10.1007/s12975-020-00806-z
Liu Y, Mi Y, Wang Y, Meng Q, Xu L, Liu Y, Zhou D, Wang Y, Liang D, Li W, Li N, Hou Y (2023) Loureirin C inhibits ferroptosis after cerebral ischemia reperfusion through regulation of the Nrf2 pathway in mice. Phytomedicine 113:154729. https://doi.org/10.1016/j.phymed.2023.154729
Chen Y, He W, Wei H, Chang C, Yang L, Meng J, Long T, Xu Q, Zhang C (2023) Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury. CNS Neurosci Ther. https://doi.org/10.1111/cns.14130
Zhang Y, Wang Y, Lou Y, Luo M, Lu Y, Li Z, Wang Y, Miao L (2018) Elabela, a newly discovered APJ ligand: similarities and differences with Apelin. Peptides 109:23–32. https://doi.org/10.1016/j.peptides.2018.09.006
Zhang Z, Tang J, Song J, Xie M, Liu Y, Dong Z, Liu X, Li X, Zhang M, Chen Y, Shi H, Zhong J (2022) Elabela alleviates ferroptosis, myocardial remodeling, fibrosis and heart dysfunction in hypertensive mice by modulating the IL-6/STAT3/GPX4 signaling. Free Radic Biol Med 181:130–142. https://doi.org/10.1016/j.freeradbiomed.2022.01.020
Xu P, Kong L, Tao C, Zhu Y, Cheng J, Li W, Shen N, Li R, Zhang C, Wang L, Zhang Y, Wang G, Liu X, Sun W, Hu W (2023) Elabela-APJ axis attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal ferroptosis. Free Radic Biol Med 196:171–186. https://doi.org/10.1016/j.freeradbiomed.2023.01.008
Tuo QZ, Liu Y, Xiang Z, Yan HF, Zou T, Shu Y, Ding XL, Zou JJ, Xu S, Tang F, Gong YQ, Li XL, Guo YJ, Zheng ZY, Deng AP, Yang ZZ, Li WJ, Zhang ST, Ayton S, Bush AI, Xu H, Dai L, Dong B, Lei P (2022) Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther 7:59. https://doi.org/10.1038/s41392-022-00917-z
Li M, Meng Z, Yu S, Li J, Wang Y, Yang W, Wu H (2022) Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem Biol Interact 366:110137. https://doi.org/10.1016/j.cbi.2022.110137
Wang Z, Li Y, Ye Y, Zhu H, Zhang J, Wang H, Lei J, Gu L, Zhan L (2023) NLRP3 inflammasome deficiency attenuates cerebral ischemia-reperfusion injury by inhibiting ferroptosis. Brain Res Bull 193:37–46. https://doi.org/10.1016/j.brainresbull.2022.11.016
Wu X, Wang B, Zhou Y, Yang Z, Jiang L, Kou Z, Li J, Ma X, Song J (2023) NLRP3 inflammasome inhibitor MCC950 reduces cerebral ischemia/reperfusion induced neuronal ferroptosis. Neurosci Lett 795:137032. https://doi.org/10.1016/j.neulet.2022.137032
Yu C, Chen P, Miao L, Di G (2023) The role of the NLRP3 inflammasome and programmed cell death in acute liver injury. Int J Mol Sci. https://doi.org/10.3390/ijms24043067
Xu Y, Liu Y, Li K, Yuan D, Yang S, Zhou L, Zhao Y, Miao S, Lv C, Zhao J (2022) COX-2/PGE2 pathway inhibits the ferroptosis induced by cerebral ischemia reperfusion. Mol Neurobiol 59:1619–1631. https://doi.org/10.1007/s12035-021-02706-1
Jin X, Jiang C, Zou Z, Huang H, Li X, Xu S, Tan R (2023) Ferritinophagy in the etiopathogenic mechanism of related diseases. J Nutr Biochem 117:109339. https://doi.org/10.1016/j.jnutbio.2023.109339
Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao Z, Zhao P, Miao Z, Zhao L, Hu J, You Y, Liu N, Chao H, Ji J (2021) Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res 174:105933. https://doi.org/10.1016/j.phrs.2021.105933
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
LXY: Conceptualization, Modification, Visualization, Writing—original draft, Writing—review and editing. CP: Conceptualization, Supervision. MLY: Software, Visualization. YXY: Literature checking. YCQ: Literature checking. DGH: Conceptualization, Supervision, Writing—review and editing. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luan, X., Chen, P., Miao, L. et al. Ferroptosis in organ ischemia–reperfusion injuries: recent advancements and strategies. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04978-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04978-2