Abstract
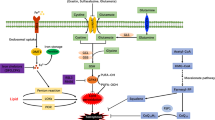
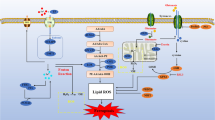
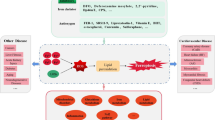
Ferroptosis, a newly discovered form of regulated cell death dependent on iron and reactive oxygen species, is mainly characterized by mitochondrial shrinkage, increased density of bilayer membranes and the accumulation of lipid peroxidation, causing membrane lipid peroxidation and eventually cell death. Similar with the most forms of regulated cell death, ferroptosis also participated in the pathological metabolism of myocardial infarction and myocardial ischemia/reperfusion injuries, which are still the leading causes of death worldwide. Given the crucial roles ferroptosis played in cardiovascular diseases, such as myocardial infarction and myocardial ischemia/reperfusion injuries, it is considerable to delve into the molecular mechanisms of ferroptosis contributing to the progress of cardiovascular diseases, which might offer the potential role of ferroptosis as a targeted treatment for a wide range of cardiovascular diseases. This review systematically summarizes the process and regulatory metabolisms of ferroptosis, discusses the relationship between ferroptosis and myocardial infarction as well as myocardial ischemia/reperfusion injuries, which might potentially provide novel insights for the pathological metabolism and original ideas for the prevention as well as treatment targeting ferroptosis of cardiovascular diseases such as myocardial infarction and myocardial ischemia/reperfusion injuries.


Similar content being viewed by others
Data availability
All the data generated during this study are included in this article and are available upon reasonable request to the first author.
Abbreviations
- RCD:
-
Regulated cell death
- MI:
-
Myocardial infarction
- MIRI:
-
Myocardial ischemia/reperfusion injuries
- CVDs:
-
Cardiovascular diseases
- HF:
-
Heart failure
- ROS:
-
Reactive oxygen species
- RSL3:
-
RAS-selective lethal compound 3
- PUFAs:
-
Polyunsaturated fatty acids
- MUFAs:
-
Monounsaturated fatty acids
- ACSL4:
-
Acyl-CoA synthetase long-chain family member 4
- LPCAT3:
-
Lysophosphatidyl choline acyltransferase 3
- LOXs:
-
Lipoxygenases
- Lip:
-
Labile iron pool
- GPX4:
-
Glutathione peroxidase 4
- DMT1:
-
Divalent metal transporter 1
- AGPS:
-
Alkyl glycerol phosphate synthase
- FAR1:
-
Fatty acyl-CoA reductase 1
- GNPAT:
-
Glyceronephosphate O-acyltransferase
- AGPAT3:
-
1-Acylglycerol-3-phosphate O-acyltransferase 3
- ER:
-
Endoplasmic reticulum
- PEDS1:
-
Plasmanylethanolamine desaturase 1
- Fe2+ :
-
Ferrous iron
- Fe3+ :
-
Ferric iron
- TF:
-
Transferrin
- TFR1:
-
Transferrin receptor 1
- STEAP3:
-
Six-transmembrane epithelial antigens of the prostate 3
- FPN1:
-
Feroportin-1
- NCOA4:
-
Nuclear receptor coactivator 4
- HO-1:
-
Heme oxygenase-1
- CO:
-
Carbon monoxide
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- KEAP1:
-
Kelch-like ECH-associated protein 1
- ARE:
-
Antioxidant response element
- FTL:
-
The light chain of ferritin
- FTH1:
-
The heavy chain of ferritin
- FPN:
-
Ferroportin
- HSPs:
-
Heat shock proteins
- TRIM21:
-
Tripartite motif containing 21
- HSPB1:
-
Heat shock protein beta-1
- IREB-2:
-
Iron-responsive element-binding protein-2
- CP:
-
Ceruloplasmin
- LTF:
-
Lactose transferrin
- PROM2:
-
Prominin-2
- ATP:
-
Adenosine triphosphate
- ISCs:
-
Iron-sulfur clusters
- OXPHOS:
-
Oxidative phosphorylation
- NOX4:
-
NADPH oxidase 4
- TCA:
-
Tricarboxylic acid
- MMP:
-
Mitochondrial membrane potential
- ETC:
-
Electron transfer chain
- Fer-1:
-
Ferrostatin-1
- SLC3A2:
-
Solute carrier family 3 member 2
- SLC7A11:
-
Catalytic subunit solute carrier family 7 member 11
- Se:
-
Selenium
- Sec:
-
Selenocysteine
- SAT1:
-
Spermidine/spermine N1-acetyltransferase 1
- ALOX-15:
-
Arachidonate lipoxygenase 15
- ATF3:
-
Activating transcription factor 3
- MVA:
-
Mevalonate
- IPP:
-
Isopentenyl pyrophosphate
- FSP1:
-
Ferroptosis suppressor protein 1
- CoQ10:
-
Coenzyme Q10
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- AIFM2:
-
Apoptosis-inducing factor mitochondrial 2
- ESCRT:
-
Endosomal sorting complexes required for transport
- PPARα:
-
Peroxisome proliferator-activated receptor α
- MEG3:
-
Maternally-expressed gene 3
- miR-214:
-
MicroRNA-214
- GCH1:
-
GTP cyclohydrolase-1
- BH4/BH2:
-
Tetrahydrobiopterin/dihydrobiopterin
- RTAs:
-
Radical-trapping antioxidants
- ox-LDLs:
-
Oxidized low-density lipoproteins
- BACH1:
-
BTB and CNC homology 1
- HUCB-MSCs:
-
Human umbilical cord blood-derived MSC exosomes
- MLK3:
-
Mixed lineage kinase 3
- CABG:
-
Coronary artery bypass grafting
- MDA:
-
Malondialdehyde
- ERS:
-
Endoplasmic reticulum stress
- LVEF:
-
Left ventricular ejection fraction
- OxPCs:
-
Oxidized phosphatidylcholines
- USP22:
-
Ubiquitin-specific peptidase 22
- LAD:
-
Left anterior descending coronary artery
- USP7:
-
Ubiquitin-specific protease 7
- ELAVL1:
-
Embryonic lethal-abnormal vision like protein 1: FOXC1: Forkhead box protein C1
- H/R:
-
Hypoxia and reoxygenation
- ERS:
-
Endoplasmic reticulum stress
- DXZ:
-
Dexrazoxane
- Lip-1:
-
Liproxstatin-1
- HO-1:
-
Heme oxygenase-1
- C3G:
-
Cyanidin-3-glucoside
- CAD:
-
Coronary artery disease
- DAMPs:
-
Damage-associated molecular patterns
References
Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R et al (2007) European guidelines on cardiovascular disease prevention in clinical practice: executive summary: fourth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 28(19):2375–2414
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ et al (2019) 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 140(11):e563–e595
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion–from mechanism to translation. Nat Med 17(11):1391–1401
Kist M, Vucic D (2021) Cell death pathways: intricate connections and disease implications. EMBO J 40(5):e106700
Schwabe RF, Luedde T (2018) Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol 15(12):738–752
Koren E, Fuchs Y (2021) Modes of regulated cell death in cancer. Cancer Discov 11(2):245–265
Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q (2021) Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res 166:105466
Tang D, Chen X, Kang R, Kroemer G (2021) Ferroptosis: molecular mechanisms and health implications. Cell Res 31(2):107–125
Chen X, Li J, Kang R, Klionsky DJ, Tang D (2021) Ferroptosis: machinery and regulation. Autophagy 17(9):2054–2081
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Wu X, Li Y, Zhang S, Zhou X (2021) Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 11(7):3052–3059
Wu D, Chen L (2015) Ferroptosis: a novel cell death form will be a promising therapy target for diseases. Acta Biochim Biophys Sin 47(10):857–859
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ et al (2017) Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285
Stockwell BR, Jiang X, Gu W (2020) Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol 30(6):478–490
Qiu Y, Cao Y, Cao W, Jia Y, Lu N (2020) The application of ferroptosis in diseases. Pharmacol Res 159:104919
Zheng J, Conrad M (2020) The metabolic underpinnings of ferroptosis. Cell Metab 32(6):920–937
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I et al (2019) FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575(7784):693–698
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113(34):E4966–E4975
Xiao M, Zhong H, Xia L, Tao Y, Yin H (2017) Pathophysiology of mitochondrial lipid oxidation: role of 4-hydroxynonenal (4-HNE) and other bioactive lipids in mitochondria. Free Radical Biol Med 111:316–327
Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J et al (2019) Stearoyl-CoA Desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Can Res 79(20):5355–5366
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I et al (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98
Dixon SJ, Winter GE, Musavi LS, Lee ED, Snijder B, Rebsamen M et al (2015) Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol 10(7):1604–1609
Yuan H, Li X, Zhang X, Kang R, Tang D (2016) Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun 478(3):1338–1343
Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS et al (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90
Seiler A, Schneider M, Förster H, Roth S, Wirth EK, Culmsee C et al (2008) Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab 8(3):237–248
Chu B, Kon N, Chen D, Li T, Liu T, Jiang L et al (2019) ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat Cell Biol 21(5):579–591
Ou Y, Wang S-J, Li D, Chu B, Gu W (2016) Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci USA 113(44):E6806–E6812
Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P et al (2020) Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 585(7826):603–608
Tang D, Kroemer G (2020) Peroxisome: the new player in ferroptosis. Signal Transduct Target Ther 5(1):273
Bairwa G, Caza M, Horianopoulos L, Hu G, Kronstad J (2019) Role of clathrin-mediated endocytosis in the use of heme and hemoglobin by the fungal pathogen cryptococcus neoformans. Cell Microbiol 21(3):e12961
Lillo-Moya J, Rojas-Solé C, Muñoz-Salamanca D, Panieri E, Saso L, Rodrigo R (2021) Targeting ferroptosis against ischemia/reperfusion cardiac injury. Antioxidants 10(5):667
Chen X, Kang R, Kroemer G, Tang D (2021) Ferroptosis in infection, inflammation, and immunity. J Exp Med. https://doi.org/10.1084/jem.20210518
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X et al (2016) Ferroptosis: process and function. Cell Death Differ 23(3):369–379
Lakhal-Littleton S, Wolna M, Chung YJ, Christian HC, Heather LC, Brescia M et al (2016) An essential cell-autonomous role for hepcidin in cardiac iron homeostasis. Elife. https://doi.org/10.7554/eLife.19804
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282
Liang D, Minikes AM, Jiang X (2022) Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol Cell 82(12):2215–2227
Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL et al (2018) Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci 22(12):3826–3836
Ravingerová T, Kindernay L, Barteková M, Ferko M, Adameová A, Zohdi V et al (2020) The molecular mechanisms of iron metabolism and its role in cardiac dysfunction and cardioprotection. Int J Mol Sci 21(21):7889
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate ferroptosis. Mol Cell 59(2):298–308
Dowdle WE, Nyfeler B, Nagel J, Elling RA, Liu S, Triantafellow E et al (2014) Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat Cell Biol 16(11):1069–1079
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509(7498):105–109
Santana-Codina N, Gikandi A, Mancias JD (2021) The Role of NCOA4-mediated ferritinophagy in ferroptosis. Adv Exp Med Biol 1301:41–57
Santana-Codina N, Mancias JD (2018) The Role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals 11(4):114
Guo Y, Lu C, Hu K, Cai C, Wang W (2022) Ferroptosis in cardiovascular diseases: current status, challenges, and future perspectives. Biomolecules 12(3):390
Fang X, Wang H, Han D, Xie E, Yang X, Wei J et al (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA 116(7):2672–2680
Gao Z, Zhang Z, Gu D, Li Y, Zhang K, Dong X et al (2022) Hemin mitigates contrast-induced nephropathy by inhibiting ferroptosis via HO-1/Nrf2/GPX4 pathway. Clin Exp Pharmacol Physiol. https://doi.org/10.1111/1440-1681.13673
Adedoyin O, Boddu R, Traylor A, Lever JM, Bolisetty S, George JF et al (2018) Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am J Physiol Renal Physiol 314(5):F702–F714
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R et al (2016) Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 63(1):173–184
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23:101107
Hou K, Shen J, Yan J, Zhai C, Zhang J, Pan J-A et al (2021) Loss of TRIM21 alleviates cardiotoxicity by suppressing ferroptosis induced by the chemotherapeutic agent doxorubicin. EBioMedicine 69:103456
Xu X, Wei Y, Hua H, Jing X, Zhu H, Xiao K et al (2022) Polyphenols sourced from thunb relieve intestinal injury via modulating ferroptosis in weanling piglets under oxidative stress. Antioxidants 11(5):996
Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X et al (2015) HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene 34(45):5617–5625
Gammella E, Recalcati S, Rybinska I, Buratti P, Cairo G (2015) Iron-induced damage in cardiomyopathy: oxidative-dependent and independent mechanisms. Oxid Med Cell Longev 2015:230182
Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P et al (2020) Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 127(4):486–501
Wang P, Cui Y, Ren Q, Yan B, Zhao Y, Yu P et al (2021) Mitochondrial ferritin attenuates cerebral ischaemia/reperfusion injury by inhibiting ferroptosis. Cell Death Dis 12(5):447
Shang Y, Luo M, Yao F, Wang S, Yuan Z, Yang Y (2020) Ceruloplasmin suppresses ferroptosis by regulating iron homeostasis in hepatocellular carcinoma cells. Cell Signal 72:109633
Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ et al (2019) Prominin2 drives ferroptosis resistance by stimulating iron export. Develop Cell 51(5):575–586
Otasevic V, Vucetic M, Grigorov I, Martinovic V, Stancic A (2021) Ferroptosis in different pathological contexts seen through the eyes of mitochondria. Oxid Med Cell Longev 2021:5537330
Chang L-C, Chiang S-K, Chen S-E, Yu Y-L, Chou R-H, Chang W-C (2018) Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett 416:124–137
Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB et al (2019) Role of Mitochondria in Ferroptosis. Mol Cell 73(2):354–363
Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, Okabe K et al (2020) Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. https://doi.org/10.1172/jci.insight.132747
Jelinek A, Heyder L, Daude M, Plessner M, Krippner S, Grosse R et al (2018) Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radical Biol Med 117:45–57
Gaschler MM, Hu F, Feng H, Linkermann A, Min W, Stockwell BR (2018) Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem Biol 13(4):1013–1020
Song X, Liu J, Kuang F, Chen X, Zeh HJ, Kang R et al (2021) PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep 34(8):108767
Liu J, Kang R, Tang D (2021) Metabolic checkpoint of ferroptosis resistance. Mol Cell Oncol 8(3):1901558
Zhou B, Liu J, Kang R, Klionsky DJ, Kroemer G, Tang D (2020) Ferroptosis is a type of autophagy-dependent cell death. Semin Cancer Biol 66:89–100
Zheng K, Dong Y, Yang R, Liang Y, Wu H, He Z (2021) Regulation of ferroptosis by bioactive phytochemicals: implications for medical nutritional therapy. Pharmacol Res 168:105580
Feng H, Stockwell BR (2018) Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol 16(5):e2006203
Conrad M, Proneth B (2019) Broken hearts: iron overload, ferroptosis and cardiomyopathy. Cell Res 29(4):263–264
Wang L, Liu Y, Du T, Yang H, Lei L, Guo M et al (2020) ATF3 promotes erastin-induced ferroptosis by suppressing system Xc. Cell Death Differ 27(2):662–675
Chen H, Cao L, Han K, Zhang H, Cui J, Ma X et al (2022) Patulin disrupts SLC7A11-cystine-cysteine-GSH antioxidant system and promotes renal cell ferroptosis both in vitro and in vivo. Food Chem Toxicol an Int J Publish British Industrial Biol Res Association. 166:113255
Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K et al (2018) Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell. https://doi.org/10.1016/j.cell.2017.11.048
Maiorino M, Conrad M, Ursini F (2018) GPx4, lipid peroxidation, and cell death: discoveries, rediscoveries, and open issues. Antioxid Redox Signal 29(1):61–74
Liu W, Zhou Y, Duan W, Song J, Wei S, Xia S et al (2021) Glutathione peroxidase 4-dependent glutathione high-consumption drives acquired platinum chemoresistance in lung cancer-derived brain metastasis. Clin Transl Med 11(9):e517
Wang Y, Zhao Y, Wang H, Zhang C, Wang M, Yang Y et al (2020) Histone demethylase KDM3B protects against ferroptosis by upregulating SLC7A11. FEBS Open Bio 10(4):637–643
Ca C, Wang D, Yu Y, Zhao T, Min N, Wu Y et al (2021) Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis 12(1):65
Asperti M, Bellini S, Grillo E, Gryzik M, Cantamessa L, Ronca R et al (2021) H-ferritin suppression and pronounced mitochondrial respiration make Hepatocellular Carcinoma cells sensitive to RSL3-induced ferroptosis. Free Radical Biol Med 169:294–303
Li S, He Y, Chen K, Sun J, Zhang L, He Y et al (2021) RSL3 drives ferroptosis through NF-B pathway activation and GPX4 depletion in Glioblastoma. Oxid Med Cell Longev 2021:2915019
Gao R, Kalathur RKR, Coto-Llerena M, Ercan C, Buechel D, Shuang S et al (2021) YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med 13(12):e14351
Yang L, Jiang L, Sun X, Li J, Wang N, Liu X et al (2022) DEHP induces ferroptosis in testes via p38α-lipid ROS circulation and destroys the BTB integrity. Food and Chem Toxicol an Int J Publish British Industrial Biol Res Association 164:113046
Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR (2018) Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci 12:466
Fan Z, Wirth AK, Chen D, Wruck CJ, Rauh M, Buchfelder M et al (2017) Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis 6(8):e371
Carpi-Santos R, Calaza KC (2018) Alterations in system x expression in the retina of Type 1 diabetic rats and the role of Nrf2. Mol Neurobiol 55(10):7941–7948
Chen D, Tavana O, Chu B, Erber L, Chen Y, Baer R et al (2017) NRF2 Is a Major Target of ARF in p53-Independent Tumor Suppression. Mol Cell 68(1):224–232
Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P et al (2018) BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 20(10):1181–1192
Zhang Y, Koppula P, Gan B (2019) Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle 18(8):773–783
Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang Z et al (2020) Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK-dependent manner. J Cell Mol Med 24(12):6670–6679
Leu JIJ, Murphy ME, George DL (2019) Mechanistic basis for impaired ferroptosis in cells expressing the African-centric S47 variant of p53. Proc Natl Acad Sci USA 116(17):8390–8396
Zhang X, Shi Z, Liu Q, Quan H, Cheng X (2019) Effects of coenzyme Q10 intervention on diabetic kidney disease: a systematic review and meta-analysis. Medicine 98(24):e15850
Hu S, Ma J, Su C, Chen Y, Shu Y, Qi Z et al (2021) Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater 135:567–581
Seibt TM, Proneth B, Conrad M (2019) Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biol Med 133:144–152
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ et al (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191
Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA et al (2018) FINO initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14(5):507–515
Hu Q, Zhang Y, Lou H, Ou Z, Liu J, Duan W et al (2021) GPX4 and vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis 12(7):706
Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E et al (2019) Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177(5):1262–1279
Yao X, Xie R, Cao Y, Tang J, Men Y, Peng H et al (2021) Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J Nanobiotechnol 19(1):311
Wu Z, Geng Y, Lu X, Shi Y, Wu G, Zhang M et al (2019) Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA 116(8):2996–3005
Zhu S, Zhang Q, Sun X, Zeh HJ, Lotze MT, Kang R et al (2017) HSPA5 regulates ferroptotic cell death in cancer cells. Can Res 77(8):2064–2077
Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH et al (2019) The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575(7784):688–692
Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D (2020) AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun 523(4):966–971
Dai E, Meng L, Kang R, Wang X, Tang D (2020) ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem Biophys Res Commun 522(2):415–421
Elguindy MM, Nakamaru-Ogiso E (2015) Apoptosis-inducing Factor (AIF) and Its family member protein, AMID, Are rotenone-sensitive NADH: ubiquinone oxidoreductases (NDH-2). J Biol Chem 290(34):20815–20826
Santoro MM (2020) The antioxidant role of non-mitochondrial CoQ10: mystery solved! Cell Metab 31(1):13–15
Venkatesh D, O’Brien NA, Zandkarimi F, Tong DR, Stokes ME, Dunn DE et al (2020) MDM2 and MDMX promote ferroptosis by PPARα-mediated lipid remodeling. Genes Dev 34(7–8):526–543
Bai T, Liang R, Zhu R, Wang W, Zhou L, Sun Y (2020) MicroRNA-214-3p enhances erastin-induced ferroptosis by targeting ATF4 in hepatoma cells. J Cell Physiol 235(7–8):5637–5648
Nguyen HP, Yi D, Lin F, Viscarra JA, Tabuchi C, Ngo K et al (2020) Aifm2, a NADH oxidase, supports robust glycolysis and is required for cold- and diet-induced thermogenesis. Mol Cell 77(3):600–617
Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F et al (2020) GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci 6(1):41–53
Soula M, Weber RA, Zilka O, Alwaseem H, La K, Yen F et al (2020) Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat Chem Biol 16(12):1351–1360
Kim HK, Han J (2020) Tetrahydrobiopterin in energy metabolism and metabolic diseases. Pharmacol Res 157:104827
Wang Y, Chen J, Li S, Zhang X, Guo Z, Hu J et al (2020) Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox Biol 32:101514
Liu P, Liu J, Wu Y, Xi W, Wei Y, Yuan Z et al (2020) Zinc supplementation protects against diabetic endothelial dysfunction via GTP cyclohydrolase 1 restoration. Biochem Biophys Res Commun 521(4):1049–1054
Abu-Taweel GM, Attia MF, Hussein J, Mekawi EM, Galal HM, Ahmed EI et al (2020) Curcumin nanoparticles have potential antioxidant effect and restore tetrahydrobiopterin levels in experimental diabetes. Biomed Pharmacotherapy Biomed Pharmacotherapie. 131:110688
Kim HK, Ko TH, Song I-S, Jeong YJ, Heo HJ, Jeong SH et al (2020) BH4 activates CaMKK2 and rescues the cardiomyopathic phenotype in rodent models of diabetes. Life Sci Alliance 3(9):e201900619
Carnicer R, Duglan D, Ziberna K, Recalde A, Reilly S, Simon JN et al (2021) BH4 increases nNOS activity and preserves left ventricular function in diabetes. Circ Res 128(5):585–601
Zhang J, Bolli R, Garry DJ, Marbán E, Menasché P, Zimmermann W-H et al (2021) Basic and translational research in cardiac repair and regeneration: JACC state-of-the-art review. J Am Coll Cardiol 78(21):2092–2105
Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN (2019) Fundamental mechanisms of regulated cell death and implications for heart disease. Physiol Rev 99(4):1765–1817
Bulluck H, Rosmini S, Abdel-Gadir A, White SK, Bhuva AN, Treibel TA et al (2016) Residual myocardial iron following intramyocardial hemorrhage during the convalescent phase of reperfused ST-segment-elevation myocardial infarction and adverse left ventricular remodeling. Circ Cardiovascular Imaging. https://doi.org/10.1161/CIRCIMAGING.116.004940
Park T-J, Park JH, Lee GS, Lee J-Y, Shin JH, Kim MW et al (2019) Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis 10(11):835
Hu H, Chen Y, Jing L, Zhai C, Shen L (2021) The link between ferroptosis and cardiovascular diseases: a novel target for treatment. Front Cardiovascular Med 8:710963
Tang L-J, Luo X-J, Tu H, Chen H, Xiong X-M, Li N-S et al (2021) Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch Pharmacol 394(2):401–410
Liu B, Zhao C, Li H, Chen X, Ding Y, Xu S (2018) Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun 497(1):233–240
Chen X, Xu S, Zhao C, Liu B (2019) Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun 516(1):37–43
Nishizawa H, Matsumoto M, Shindo T, Saigusa D, Kato H, Suzuki K et al (2020) Ferroptosis is controlled by the coordinated transcriptional regulation of glutathione and labile iron metabolism by the transcription factor BACH1. J Biol Chem 295(1):69–82
Song Y, Wang B, Zhu X, Hu J, Sun J, Xuan J et al (2021) Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol 37(1):51–64
Qin Y, Qiao Y, Wang D, Tang C, Yan G (2021) Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed Pharmacotherapy Biomed Pharmacotherapie 141:111872
Tang S, Wang Y, Ma T, Lu S, Huang K, Li Q et al (2020) MiR-30d inhibits cardiomyocytes autophagy promoting ferroptosis after myocardial infarction. Panminerva Med. https://doi.org/10.23736/S0031-0808.20.03979-8
Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M et al (2018) Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol 314(3):H659–H668
Wang K, Gan T-Y, Li N, Liu C-Y, Zhou L-Y, Gao J-N et al (2017) Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ 24(6):1111–1120
Zheng H, Shi L, Tong C, Liu Y, Hou M (2021) circSnx12 Is Involved in Ferroptosis During Heart Failure by Targeting miR-224-5p. Front Cardiovascular Med 8:656093
Wang J, Deng B, Liu Q, Huang Y, Chen W, Li J et al (2020) Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis 11(7):574
Williams RE, Zweier JL, Flaherty JT (1991) Treatment with deferoxamine during ischemia improves functional and metabolic recovery and reduces reperfusion-induced oxygen radical generation in rabbit hearts. Circulation 83(3):1006–1014
Paraskevaidis IA, Iliodromitis EK, Vlahakos D, Tsiapras DP, Nikolaidis A, Marathias A et al (2005) Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: immediate and long-term significance. Eur Heart J 26(3):263–270
Stamenkovic A, O’Hara KA, Nelson DC, Maddaford TG, Edel AL, Maddaford G et al (2021) Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 320(3):H1170–H1184
Ma S, Sun L, Wu W, Wu J, Sun Z, Ren J (2020) USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Front Physiol 11:551318
Luo E-F, Li H-X, Qin Y-H, Qiao Y, Yan G-L, Yao Y-Y et al (2021) Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes 12(2):124–137
Chen H-Y, Xiao Z-Z, Ling X, Xu R-N, Zhu P, Zheng S-Y (2021) ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med 27(1):14
Tang L-J, Zhou Y-J, Xiong X-M, Li N-S, Zhang J-J, Luo X-J et al (2021) Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radical Biol Med 162:339–352
Lee H, Zandkarimi F, Zhang Y, Meena JK, Kim J, Zhuang L et al (2020) Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat Cell Biol 22(2):225–234
Li W, Li W, Leng Y, Xiong Y, Xia Z (2020) Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol 39(2):210–225
Ni R, Cao T, Xiong S, Ma J, Fan G-C, Lacefield JC et al (2016) Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radical Biol Med 90:12–23
Zhao Z, Wu J, Xu H, Zhou C, Han B, Zhu H et al (2020) XJB-5-131 inhibited ferroptosis in tubular epithelial cells after ischemia-reperfusion injury. Cell Death Dis 11(8):629
Feng Y, Madungwe NB, Imam Aliagan AD, Tombo N, Bopassa JC (2019) Liproxstatin-1 protects the mouse myocardium against ischemia/reperfusion injury by decreasing VDAC1 levels and restoring GPX4 levels. Biochem Biophys Res Commun 520(3):606–611
Xie Y, Song X, Sun X, Huang J, Zhong M, Lotze MT et al (2016) Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem Biophys Res Commun 473(4):775–780
Li Q, Li Q-Q, Jia J-N, Sun Q-Y, Zhou H-H, Jin W-L et al (2019) Baicalein exerts neuroprotective effects in FeCl-induced posttraumatic epileptic seizures suppressing ferroptosis. Front Pharmacol 10:638
Fan Z, Cai L, Wang S, Wang J, Chen B (2021) Baicalin Prevents myocardial ischemia/reperfusion injury through inhibiting ACSL4 mediated ferroptosis. Front Pharmacol 12:628988
Lv Z, Fe W, Zhang X, Zhang X, Zhang J, Liu R (2021) Etomidate attenuates the ferroptosis in myocardial ischemia/reperfusion rat model via Nrf2/HO-1 pathway. Shock 56(3):440–449
Shan X, Lv Z-Y, Yin M-J, Chen J, Wang J, Wu Q-N (2021) The protective effect of cyanidin-3-glucoside on myocardial ischemia-reperfusion injury through ferroptosis. Oxid Med Cell Longev 2021:8880141
Tu H, Zhou Y-J, Tang L-J, Xiong X-M, Zhang X-J, Ali Sheikh MS et al (2021) Combination of ponatinib with deferoxamine synergistically mitigates ischemic heart injury via simultaneous prevention of necroptosis and ferroptosis. Eur J Pharmacol 898:173999
Tan Z, Liu H, Song X, Ling Y, He S, Yan Y et al (2019) Honokiol post-treatment ameliorates myocardial ischemia/reperfusion injury by enhancing autophagic flux and reducing intracellular ROS production. Chem Biol Interact 307:82–90
Li T, Tan Y, Ouyang S, He J, Liu L (2022) Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene 808:145968
Zhou X-R, Ru X-C, Xiao C, Pan J, Lou Y-Y, Tang L-H et al (2021) Sestrin2 is involved in the Nrf2-regulated antioxidative signaling pathway in luteolin-induced prevention of the diabetic rat heart from ischemia/reperfusion injury. Food Funct 12(8):3562–3571
Li J-Y, Yao Y-M, Tian Y-P (2021) Ferroptosis: a trigger of proinflammatory state progression to immunogenicity in necroinflammatory disease. Front Immunol 12:701163
Li J, Cao F, Yin H-L, Huang Z-J, Lin Z-T, Mao N et al (2020) Ferroptosis: past, present and future. Cell Death Dis 11(2):88
Yan B, Ai Y, Sun Q, Ma Y, Cao Y, Wang J et al (2021) Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol Cell 81(2):355–369
Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ et al (2016) Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol 12(7):497–503
Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H et al (2020) Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett 483:127–136
Chan W, Taylor AJ, Ellims AH, Lefkovits L, Wong C, Kingwell BA et al (2012) Effect of iron chelation on myocardial infarct size and oxidative stress in ST-elevation-myocardial infarction. Circ Cardiovasc Interv 5(2):270–278
Howland MA (1996) Risks of parenteral deferoxamine for acute iron poisoning. J Toxicol Clin Toxicol 34(5):491–497
Donfrancesco A, Deb G, Dominici C, Pileggi D, Castello MA, Helson L (1990) Effects of a single course of deferoxamine in neuroblastoma patients. Can Res 50(16):4929–4930
Ramos C, Brito R, González-Montero J, Valls N, Gormaz JG, Prieto JC et al (2017) Effects of a novel ascorbate-based protocol on infarct size and ventricle function in acute myocardial infarction patients undergoing percutaneous coronary angioplasty. Archives Med Sci AMS 13(3):558–567
Valls N, Gormaz JG, Aguayo R, González J, Brito R, Hasson D et al (2016) Amelioration of persistent left ventricular function impairment through increased plasma ascorbate levels following myocardial infarction. Redox Report Commun Free Radical Res 21(2):75–83
Gasparetto C, Malinverno A, Culacciati D, Gritti D, Prosperini PG, Specchia G et al (2005) Antioxidant vitamins reduce oxidative stress and ventricular remodeling in patients with acute myocardial infarction. Int J Immunopathol Pharmacol 18(3):487–496
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81870265, 82171808) and the China Young and Middle-aged Clinical Research Foundation (Grant No. 2017CCA-VG045), Foundation,2017CCA-VG045,2017CCA-VG045,2017CCA-VG045,2017CCA-VG045,2017CCA-VG045,2017CCA-VG045,2017CCA-VG045.
Author information
Authors and Affiliations
Contributions
XJH: writing—original draft preparation. XJH, JZ, JL and XJ: writing—review and editing. CXG: supervision. HXW, FHD and CXG: project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests or other competing interests.
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, X., Zhang, J., Liu, J. et al. Targeting ferroptosis: a novel insight against myocardial infarction and ischemia–reperfusion injuries. Apoptosis 28, 108–123 (2023). https://doi.org/10.1007/s10495-022-01785-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-022-01785-2