Abstract
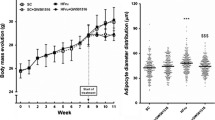
The prevalence of the metabolic syndrome (MetS) and its cardiac comorbidities as cardiac hypertrophy (CH) have increased considerably due to the high consumption of carbohydrates, such as sucrose and/or fructose. We compared the effects of sucrose (S), fructose (F) and their combination (S + F) on the development of MetS in weaned male Wistar rats and established the relationship between the consumption of these sugars and the degree of cardiac CH development, oxidative stress (OS) and Calcium/calmodulin-dependent protein kinase type II subunit delta oxidation (ox-CaMKIIδ). 12 weeks after the beginning of treatments with S, F or S + F, arterial pressure was measured and 8 weeks later (to complete 20 weeks) the animals were sacrificed and blood samples, visceral adipose tissue and hearts were obtained. Biochemical parameters were determined in serum and cardiac tissue to evaluate the development of MetS and OS. To evaluate CH, atrial natriuretic peptide (ANP), CaMKIIδ and ox-CaMKIIδ were determined by western blot and histological studies were performed in cardiac tissue. Our data showed that chronic consumption of S + F exacerbates MetS-induced CH which is related with a higher OS and ox-CaMKIIδ.





source of reactive oxygen species causing oxidative stress in different organs such as the heart. B CaMKIIδ activation is carried out by Ca2+-CaM complex binding to the regulatory domain, however, when this binding is reversed CaMKIIδ is autoinhibited. However, Ca2+-CaM complex-independent activation also occurs. Oxidation at the regulatory site of methionine 281 and 282 is a type of Ca2+-CaM-independent activation of this enzyme, moreover, it is dependent on the production of reactive oxygen species and a state of oxidative stress. This activation is known as pathological activation because it leads to the development of different abnormal conditions such as cardiac hypertrophy
Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Pepin A, Stanhope KL, Imbeault P (2019) Are fruit juices healthier than sugar-sweetened beverages? A review. Nutrients 11(5):1–16. https://doi.org/10.3390/nu11051006
Ng SW, Slining MM, Popkin BM (2012) Use of Caloric and Noncaloric Sweeteners in US Consumer Packaged Foods, 2005–2009. J Acad Nutr Diet 12(11):1828-1834.e6. https://doi.org/10.1016/j.jand.2012.07.009
Balderas-Villalobos J, Molina-Muñoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gómez-Viquez NL (2013) Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol 305(9):H1344–H1353. https://doi.org/10.1152/ajpheart.00211.2013
Feillet-Coudray C, Fouret G, Vigor C, Bonafos B, Jover B, Blachnio-Zabielska A et al (2019) Long-term measures of dyslipidemia, inflammation, and oxidative stress in rats fed a high-fat/high-fructose diet. Lipids 54(1):81–97. https://doi.org/10.1002/lipd.12128
Szűcs G, Sója A, Péter M, Sárközy M, Bruszel B, Siska A et al (2019) Prediabetes induced by fructose-enriched diet influences cardiac lipidome and proteome and leads to deterioration of cardiac function prior to the development of excessive oxidative stress and cell damage. Hindawi Oxidat Med Cell Longev 2019:3218275. https://doi.org/10.1155/2019/3218275
Zhang YB, Meng YH, Chang S, Zhang RY, Shi C (2016) High fructose causes cardiac hypertrophy via mitochondrial signaling pathway. Am J Transl Res 8(11):4869–4880
Kitagawa A, Ohta Y, Ohashi K, Yashiro K, Fukuzawa K (2020) Effect of high fructose-induced metabolic syndrome on tissue vitamin e and lipid peroxide levels in rats. J Nutr Sci Vitaminol. https://doi.org/10.3177/jnsv.66.200
Moreno-Fernández S, Garcés-Rimón M, Vera G, Astier J, Landrier JF, Miguel M (2018) High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients. https://doi.org/10.3390/nu10101502
Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D (2019) Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev 2019:8267234. https://doi.org/10.1155/2019/8267234
Aydin S, Aksoy A, Aydin S, Kalayci M, Yilmaz M, Kuloglu T et al (2014) Today’s and yesterday’s of pathophysiology: biochemistry of metabolic syndrome and animal models. Nutrition 30(1):1–9. https://doi.org/10.1016/j.nut.2013.05.013
Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K et al (2013) Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. https://doi.org/10.1038/nature12537
Erickson JR, Joiner M, Ling A, Guan X, Kutschke W, Yang J, Oddis CV et al (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. https://doi.org/10.1016/j.cell.2008.02.048
Erickson JR, Julie He B, Grumbach IM, Anderson ME (2011) CaMKII in the cardiovascular system: sensing redox states. Physiol Rev 91(3):889–915. https://doi.org/10.1152/physrev.00018.2010
Feng N, Anderson ME (2017) CaMKII is a nodal signal for multiple programmed cell death pathways in heart. J Mol Cell Cardiol 103:102–109. https://doi.org/10.1016/j.yjmcc.2016.12.007
Qu J, Mei Q, Niu R (2019) Oxidative CaMKII as a potential target for inflammatory disease (Review). Mol Med Rep 20(2):863–870. https://doi.org/10.3892/mmr.2019.10309
Zhong P, Quan D, Peng J, Xiong X, Liu Y, Kong B et al (2017) Role of CaMKII in free fatty acid/hyperlipidemia-induced cardiac remodeling both in vitro and in vivo. J Mol Cell Cardiol 109:1–16. https://doi.org/10.1016/j.yjmcc.2017.06.010
Mattiazzi A, Bassani RA, Escobar AL, Palomeque J, Valverde CA, Vila Petroff M et al (2015) Chasing cardiac physiology and pathology down the CaMKII cascade. Am J Physiol 308(10):H1177–H1191. https://doi.org/10.1152/ajpheart.00007.2015
Federico M, Portiansky EL, Sommese L, Alvarado FJ, Blanco PG, Zanuzzi CN et al (2017) Calcium-calmodulin-dependent protein kinase mediates the intracellular signalling pathways of cardiac apoptosis in mice with impaired glucose tolerance. J Physiol. https://doi.org/10.1113/JP273714
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4):1220S-1228S. https://doi.org/10.1093/ajcn/65.4.1220s
Colado-Velázquez J, Mailloux-Salinas P, Medina-Contreras J, Cruz-Robles D, Bravo G (2015) Effect of Serenoa Repens on oxidative stress, inflammatory and growth factors in obese Wistar rats with benign prostatic hyperplasia. Phyther Res 29(10):1525–1531. https://doi.org/10.1002/ptr.5406
Carvajal K, El Hafidi M, Marin-Hernández A, Moreno-Sánchez R (2005) Structural and functional changes in heart mitochondria from sucrose-fed hypertriglyceridemic rats. Biochim Biophys Acta. https://doi.org/10.1016/j.bbabio.2005.08.001
El Hafidi M, Cuéllar A, Ramírez J, Baos G (2001) Effect of sucrose addition to drinking water, that induces hypertension in the rats, on liver microsomal Δ9 and Δ5-desaturase activities. J Nutr Biochem 12:396–403. https://doi.org/10.1016/S0955-2863(01)00154-1
Fuente-Martín E, García-Cáceres C, Granado M, Sánchez-Garrido MA, Tena-Sempere M, Frago LM et al (2012) Early postnatal overnutrition increases adipose tissue accrual in response to a sucrose-enriched diet. Am J Physiol. https://doi.org/10.1152/ajpendo.00618.2011
Kim M, Do GY, Kim I (2020) Activation of the renin-angiotensin system in high fructose-induced metabolic syndrome. Korean J Physiol Pharmacol. https://doi.org/10.4196/KJPP.2020.24.4.319
Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH et al (2007) Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86(4):899–906
Baños G, Medina-Campos ON, Maldonado PD, Zamora J, Pérez I, Pavón N et al (2005) Activities of antioxidant enzymes in two stages of pathology development in sucrose-fed rats. Can J Physiol Pharmacol. https://doi.org/10.1139/y05-013
McEniery CM, Yasmin WS, Maki-Petaja K, McDonnell B, Sharman JE et al (2005) Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. https://doi.org/10.1161/01.HYP.0000165310.84801.e0
Souza Cruz EM, Bitencourt de Morais JM, Dalto da Rosa CV, da Silva SM, Comar JF, de Almeida Chuffa LG et al (2020) Long-term sucrose solution consumption causes metabolic alterations and affects hepatic oxidative stress in Wistar rats. Biol Open 9(3):bio047282. https://doi.org/10.1242/bio.047282
Sulzbacher Da Silva B, Macedo A, Paulino B, Taffarel M, Borba IG, Ortega Telles L et al (2021) High sucrose diet attenuates oxidative stress, inflammation and liver injury in thioacetamide-induced liver cirrhosis. Life Sci 267:118944. https://doi.org/10.1016/j.lfs.2020.118944
Gantner BN, LaFond KM, Bonini MG (2020) Nitric oxide in cellular adaptation and disease. Redox Biol 34:101550. https://doi.org/10.1016/j.redox.2020.101550
Wang C-H, Pandey S, Sivalingam K, Asokan Shibu M, Kuo W-W, Padma Viswanadha V et al (2021) Leech extract: a candidate cardioprotective against hypertension-induced cardiac hypertrophy and fibrosis. J Ethnopharmacol 264:113346. https://doi.org/10.1016/j.jep.2020.113346
Brady TM (2016) The role of obesity in the development of left ventricular hypertrophy among children and adolescents. Curr Hypertens Rep 18(1):3. https://doi.org/10.1007/s11906-015-0608-3
Cerrudo CS, Cavallero S, Fermepin MR, González GE, Donato M, Kouyoumdzian NM et al (2021) Cardiac natriuretic peptide profiles in chronic hypertension by single or sequentially combined renovascular and DOCA-salt treatments. Front Physiol 12:651246. https://doi.org/10.3389/fphys.2021.651246
Shimizu I, Minamino T (2016) Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 97:245–262. https://doi.org/10.1016/j.yjmcc.2016.06.001
Acknowledgements
The research was supported by CONACYT Post-graduate Fellowship No. 456311.
Funding
Funding was provided by Consejo Nacional de Ciencia y Tecnología (456311).
Author information
Authors and Affiliations
Contributions
GB and NLGV contributed to the study design. DJAC, PMS conceived experiments and analyzed data. DJAC and JA contributed to helpful discussion and FH reviewed the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arias-Chávez, D.J., Mailloux-Salinas, P., Altamirano, J. et al. Consumption of combined fructose and sucrose diet exacerbates oxidative stress, hypertrophy and CaMKIIδ oxidation in hearts from rats with metabolic syndrome. Mol Cell Biochem 477, 1309–1320 (2022). https://doi.org/10.1007/s11010-022-04364-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04364-w