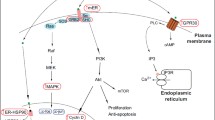
Abstract
Uterine leiomyoma is the most common tumor of the female reproductive system and originates from a single transformed myometrial smooth muscle cell. Despite the immense medical, psychosocial, and financial impact, the exact underlying mechanisms of leiomyoma pathobiology are poorly understood. Alterations of signaling pathways are thought to be instrumental in leiomyoma biology. Wnt/β-catenin pathway appears to be involved in several aspects of the genesis of leiomyomas. For example, Wnt5b is overexpressed in leiomyoma, and the Wnt/β-catenin pathway appears to mediate the role of MED12 mutations, the most common mutations in leiomyoma, in tumorigenesis. Moreover, Wnt/β-catenin pathway plays a paracrine role where estrogen/progesterone treatment of mature myometrial or leiomyoma cells leads to increased expression of Wnt11 and Wnt16, which induces proliferation of leiomyoma stem cells and tumor growth. Constitutive activation of β-catenin leads to myometrial hyperplasia and leiomyoma-like lesions in animal models. Wnt/β-catenin signaling is also closely involved in mechanotransduction and extracellular matrix regulation and relevant alterations in leiomyoma, and crosstalk is noted between Wnt/β-catenin signaling and other pathways known to regulate leiomyoma development and growth such as estrogen, progesterone, TGFβ, PI3K/Akt/mTOR, Ras/Raf/MEK/ERK, IGF, Hippo, and Notch signaling. Finally, evidence suggests that inhibition of the canonical Wnt pathway using β-catenin inhibitors inhibits leiomyoma cell proliferation. Understanding the molecular mechanisms of leiomyoma development is essential for effective treatment. The specific Wnt/β-catenin pathway molecules discussed in this review constitute compelling candidates for therapeutic targeting.





Similar content being viewed by others
Data availability
No new data were generated or analyzed in support of this research.
References
Fortin C, Flyckt R, Falcone T (2018) Alternatives to hysterectomy: the burden of fibroids and the quality of life. Best Pract Res Clin Obstet Gynaecol 46:31–42. https://doi.org/10.1016/j.bpobgyn.2017.10.001
Gupta S, Jose J, Manyonda I (2008) Clinical presentation of fibroids. Best Pract Res Clin Obstet Gynaecol 22(4):615–626
Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH (2012) The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 206(3):211.e1-211.e9. https://doi.org/10.1016/j.ajog.2011.12.002
Fritton K, Borahay MA (2017) New and emerging therapies for uterine fibroids. Semin Reprod Med 35(6):549–559. https://doi.org/10.1055/s-0037-1606303
Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T et al (2007) Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci 104(47):18700–18705. https://doi.org/10.1073/pnas.0704472104
Chang HL, Senaratne TN, Zhang L, Szotek PP, Stewart E, Dombkowski D et al (2010) Uterine leiomyomas exhibit fewer stem/progenitor cell characteristics when compared with corresponding normal myometrium. Reprod Sci 17(2):158–167. https://doi.org/10.1177/1933719109348924
Ono M, Qiang W, Serna VA, Yin P, Coon JS, Navarro A et al (2012) Role of stem cells in human uterine leiomyoma growth. PLoS One 7(5):e36935. https://doi.org/10.1371/journal.pone.0036935
Mas A, Cervello I, Gil-Sanchis C, Faus A, Ferro J, Pellicer A et al (2012) Identification and characterization of the human leiomyoma side population as putative tumor-initiating cells. Fertil Steril 98(3):741-751.e6. https://doi.org/10.1016/j.fertnstert.2012.04.044
Mas A, Nair S, Laknaur A, Simon C, Diamond MP, Al-Hendy A (2015) Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril 104(1):225-234.e3. https://doi.org/10.1016/j.fertnstert.2015.04.021
Yin P, Ono M, Moravek MB, Coon JS, Navarro A, Monsivais D et al (2015) Human uterine leiomyoma stem/progenitor cells expressing CD34 and CD49b initiate tumors in vivo. J Clin Endocrinol Metab 100(4):E601–E606. https://doi.org/10.1210/jc.2014-2134
Patterson AL, George JW, Chatterjee A, Carpenter TJ, Wolfrum E, Chesla DW et al (2020) Putative human myometrial and fibroid stem-like cells have mesenchymal stem cell and endometrial stromal cell properties. Hum Reprod 35(1):44–57. https://doi.org/10.1093/humrep/dez247
Islam MS, Ciavattini A, Petraglia F, Castellucci M, Ciarmela P (2018) Extracellular matrix in uterine leiomyoma pathogenesis: a potential target for future therapeutics. Hum Reprod Update 24(1):59–85. https://doi.org/10.1093/humupd/dmx032
Leppert PC, Jayes FL, Segars JH (2014) The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int 2014:783289. https://doi.org/10.1155/2014/783289
Corachán A, Ferrero H, Aguilar A, Garcia N, Monleon J, Faus A et al (2019) Inhibition of tumor cell proliferation in human uterine leiomyomas by vitamin D via Wnt/β-catenin pathway. Fertil Steril 111(2):397–407
Shen Y, Lu Q, Zhang P, Wu Y, Ren M (2018) The effect of TGF-β signaling on regulating proliferation of uterine leiomyoma cell via ERα signaling activated by bisphenol A, octylphenol and nonylphenol in vitro. J Cancer Res Ther 14(9):276
Zhu Y, Xu J, Li Z, Xie S, Zhou J, Guo X et al (2015) Ginsenoside Rh2 suppresses growth of uterine leiomyoma in vitro and in vivo and may regulate ERα/c-Src/p38 MAPK activity. J Funct Foods 18:73–82
Purdy MP, Ducharme M, Haak AJ, Ravix J, Tan Q, Sicard D et al (2020) YAP/TAZ are activated by mechanical and hormonal stimuli in myometrium and exhibit increased baseline activation in uterine fibroids. Reprod Sci 27(4):1074–1085. https://doi.org/10.1007/s43032-019-00106-4
Makker A, Goel MM, Mahdi AA, Bhatia V, Das V, Agarwal A et al (2016) PI3K/Akt/mTOR signaling & its regulator tumour suppressor genes PTEN & LKB1 in human uterine leiomyomas. Indian J Med Res 143:S112–S119. https://doi.org/10.4103/0971-5916.191808
Malik M, Britten JL, Catherino W (2019) IL6 and STAT-3 pathway highlight the differences in molecular responses in myometrium and uterine fibroids. Fertil Steril 112(3):e350
Borahay MA, Al-Hendy A, Kilic GS, Boehning D (2015) Signaling pathways in leiomyoma: understanding pathobiology and implications for therapy. Mol Med. https://doi.org/10.2119/molmed.2014.00053
Ono M, Yin P, Navarro A, Moravek MB, Coon JS, Druschitz SA et al (2013) Paracrine activation of WNT/beta-catenin pathway in uterine leiomyoma stem cells promotes tumor growth. Proc Natl Acad Sci 110(42):17053–8. https://doi.org/10.1073/pnas.1313650110
Ali M, Shahin SM, Sabri NA, Al-Hendy A, Yang Q (2020) Activation of β-catenin signaling and its crosstalk with estrogen and histone deacetylases in human uterine fibroids. J Clin Endocrinol Metab 105(4):e1517–e1535
Gruber J, Yee Z, Tolwinski NS (2016) Developmental drift and the role of Wnt signaling in aging. Cancers 8(8):73. https://doi.org/10.3390/cancers8080073
Kaur P, Jin HJ, Lusk JB, Tolwinski NS (2018) Modeling the role of Wnt signaling in human and drosophila stem cells. Genes 9(2):101. https://doi.org/10.3390/genes9020101
Ng LF, Kaur P, Bunnag N, Suresh J, Sung ICH, Tan QH et al (2019) WNT signaling in disease. Cells 8(8):826
Nusse R, Clevers H (2017) Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169(6):985–999
Martin-Orozco E, Sanchez-Fernandez A, Ortiz-Parra I, Ayala-San NM (2019) WNT signaling in tumors: the way to evade drugs and immunity. Front Immunol 10:2854. https://doi.org/10.3389/fimmu.2019.02854
Zhan T, Rindtorff N, Boutros M (2017) Wnt signaling in cancer. Oncogene 36(11):1461–1473
Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4(2):68–75
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11(24):3286–3305. https://doi.org/10.1101/gad.11.24.3286
Pfister AS, Kühl M (2018) Of Wnts and ribosomes. In: Teplow D (ed) Progress in molecular biology and translational science. Elsevier, Amsterdam, pp 131–155
Habas R, Dawid IB (2005) Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol 4(1):2
Minde DP, Anvarian Z, Rüdiger SG, Maurice MM (2011) Messing up disorder: how do missense mutations in the tumor suppressor protein APC lead to cancer? Mol Cancer 10(1):101
Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Iii WLP et al (1997) The mouse fused locus encodes axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90(1):181–192
Cruciat C-M (2014) Casein kinase 1 and Wnt/β-catenin signaling. Curr Opin Cell Biol 31:46–55
Wu D, Pan W (2010) GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 35(3):161–168. https://doi.org/10.1016/j.tibs.2009.10.002
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17(1):9–26. https://doi.org/10.1016/j.devcel.2009.06.016
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y et al (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108(6):837–847. https://doi.org/10.1016/s0092-8674(02)00685-2
Rao TP, Kuhl M (2010) An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 106(12):1798–1806. https://doi.org/10.1161/CIRCRESAHA.110.219840
van Amerongen R (2012) Alternative Wnt pathways and receptors. Cold Spring Harb Perspect Biol 4(10):a007914–a007914. https://doi.org/10.1101/cshperspect.a007914
VanderVorst K, Dreyer CA, Konopelski SE, Lee H, Ho HH, Carraway KL 3rd (2019) Wnt/PCP signaling contribution to carcinoma collective cell migration and metastasis. Cancer Res 79(8):1719–1729. https://doi.org/10.1158/0008-5472.CAN-18-2757
De A (2011) Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin 43(10):745–756. https://doi.org/10.1093/abbs/gmr079
Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW et al (2015) Alternative Wnt signaling activates YAP/TAZ. Cell 162(4):780–794. https://doi.org/10.1016/j.cell.2015.07.013
Gujral TS, Chan M, Peshkin L, Sorger PK, Kirschner MW, MacBeath G (2014) A noncanonical Frizzled2 pathway regulates epithelial-mesenchymal transition and metastasis. Cell 159(4):844–856. https://doi.org/10.1016/j.cell.2014.10.032
Green J, Nusse R, van Amerongen R (2014) The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol 6(2):a009175–a009175. https://doi.org/10.1101/cshperspect.a009175
Cruciat C-M, Niehrs C (2013) Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol 5(3):a015081https://doi.org/10.1101/cshperspect.a015081
Ko Y-A, Jamaluddin MFB, Adebayo M, Bajwa P, Scott RJ, Dharmarajan AM et al (2018) Extracellular matrix (ECM) activates β-catenin signaling in uterine fibroids. Reproduction 155(1):61–71
Kwong LN, Dove WF (2009) APC and its modifiers in colon cancer. Adv Exp Med Biol 656:85–106. https://doi.org/10.1007/978-1-4419-1145-2_8
Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H et al (1991) Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66(3):589–600. https://doi.org/10.1016/0092-8674(81)90021-0
Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61(5):759–767. https://doi.org/10.1016/0092-8674(90)90186-i
Caldwell CM, Kaplan KB (2009) The role of APC in mitosis and in chromosome instability. Adv Exp Med Biol 656:51–64. https://doi.org/10.1007/978-1-4419-1145-2_5
Hulsken J, Birchmeier W, Behrens J (1994) E-cadherin and APC compete for the interaction with beta-catenin and the cytoskeleton. J Cell Biol 127(6 Pt 2):2061–2069. https://doi.org/10.1083/jcb.127.6.2061
Juanes MA, Bouguenina H, Eskin JA, Jaiswal R, Badache A, Goode BL (2017) Adenomatous polyposis coli nucleates actin assembly to drive cell migration and microtubule-induced focal adhesion turnover. J Cell Biol 216(9):2859–2875. https://doi.org/10.1083/jcb.201702007
Salahshor S, Woodgett JR (2005) The links between axin and carcinogenesis. J Clin Pathol 58(3):225–236. https://doi.org/10.1136/jcp.2003.009506
Machin P, Catasus L, Pons C, Munoz J, Matias-Guiu X, Prat J (2002) CTNNB1 mutations and beta-catenin expression in endometrial carcinomas. Hum Pathol 33(2):206–212. https://doi.org/10.1053/hupa.2002.30723
Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP (2009) Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology 49(3):821–831. https://doi.org/10.1002/hep.22695
Jung YS, Park JI (2020) Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp Mol Med 52(2):183–191. https://doi.org/10.1038/s12276-020-0380-6
Lobel MK, Somasundaram P, Morton CC (2006) The genetic heterogeneity of uterine leiomyomata. Obstet Gynecol Clin North Am 33(1):13–39. https://doi.org/10.1016/j.ogc.2005.12.006
Linder D, Gartler SM (1965) Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science 150(3692):67–69
Al-Hendy A, Laknaur A, Diamond MP, Ismail N, Boyer TG, Halder SK (2017) Silencing Med12 gene reduces proliferation of human leiomyoma cells mediated via Wnt/β-catenin signaling pathway. Endocrinology 158(3):592–603
Bulun SE (2013) Uterine fibroids. N Engl J Med 369(14):1344–1355
Zaitseva M, Holdsworth-Carson SJ, Waldrip L, Nevzorova J, Martelotto L, Vollenhoven BJ et al (2013) Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction 146(2):91–102. https://doi.org/10.1530/REP-13-0087
Ono M, Yin P, Navarro A, Moravek MB, Coon VJ, Druschitz SA et al (2014) Inhibition of canonical WNT signaling attenuates human leiomyoma cell growth. Fertil Steril 101(5):1441–1449. https://doi.org/10.1016/j.fertnstert.2014.01.017
Tai CT, Lin WC, Chang WC, Chiu TH, Chen GT (2003) Classical cadherin and catenin expression in normal myometrial tissues and uterine leiomyomas. Mol Reprod Dev 64(2):172–178. https://doi.org/10.1002/mrd.10248
Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J (2012) MED12 mutations in uterine fibroids–their relationship to cytogenetic subgroups. Int J Cancer 131(7):1528–1536. https://doi.org/10.1002/ijc.27424
Mangioni S, Viganò P, Lattuada D, Abbiati A, Vignali M, Di Blasio AM (2005) Overexpression of the Wnt5b gene in leiomyoma cells: implications for a role of the Wnt signaling pathway in the uterine benign tumor. J Clin Endocrinol Metab 90(9):5349–5355
Fukuhara K, Kariya M, Kita M, Shime H, Kanamori T, Kosaka C et al (2002) Secreted frizzled related protein 1 is overexpressed in uterine leiomyomas, associated with a high estrogenic environment and unrelated to proliferative activity. J Clin Endocrinol Metab 87(4):1729–1736
El Sabeh M, Afrin S, Singh B, Miyashita-Ishiwata M, Borahay M (2020) Uterine stem cells and benign gynecological disorders: role in pathobiology and therapeutic implications. Stem Cell Rev Rep. https://doi.org/10.1007/s12015-020-10075-w
Mas A, Cervello I, Fernandez-Alvarez A, Faus A, Diaz A, Burgues O et al (2015) Overexpression of the truncated form of high mobility group A proteins (HMGA2) in human myometrial cells induces leiomyoma-like tissue formation. Mol Hum Reprod 21(4):330–338. https://doi.org/10.1093/molehr/gau114
Orciani M, Caffarini M, Biagini A, Lucarini G, Delli Carpini G, Berretta A et al (2018) Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int 2018:1716246. https://doi.org/10.1155/2018/1716246
Liu S, Yin P, Dotts AJ, Kujawa SA, Coon VJ, Wei JJ et al (2020) Activation of protein kinase B by WNT4 as a regulator of uterine leiomyoma stem cell function. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2020.06.045
Tanwar PS, Lee H-J, Zhang L, Zukerberg LR, Taketo MM, Rueda BR et al (2009) Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod 81(3):545–552
Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J (2005) Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 288(1):276–283. https://doi.org/10.1016/j.ydbio.2005.09.045
Chen HY, Huang TC, Lin LC, Shieh TM, Wu CH, Wang KL et al (2018) Fucoidan inhibits the proliferation of leiomyoma cells and decreases extracellular matrix-associated protein expression. Cell Physiol Biochem 49(5):1970–1986. https://doi.org/10.1159/000493660
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R et al (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci 96(10):5522–5527. https://doi.org/10.1073/pnas.96.10.5522
Yochum GS, Sherrick CM, Macpartlin M, Goodman RH (2010) A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5’ and 3’ Wnt responsive enhancers. Proc Natl Acad Sci 107(1):145–150. https://doi.org/10.1073/pnas.0912294107
Guo L, Yilamu D, Sun L, Liu S, Ma F (2015) Association among the expression of beta-catenin, cyclin D1 and estrogen receptor-beta in human breast cancer. Exp Ther Med 10(4):1423–1428. https://doi.org/10.3892/etm.2015.2657
Rennoll S, Yochum G (2015) Regulation of MYC gene expression by aberrant Wnt/beta-catenin signaling in colorectal cancer. World J Biol Chem 6(4):290–300. https://doi.org/10.4331/wjbc.v6.i4.290
Vadlakonda L, Pasupuleti M, Pallu R (2013) Role of PI3K-AKT-mTOR and Wnt signaling pathways in transition of g1-s phase of cell cycle in cancer cells. Front Oncol 3:85. https://doi.org/10.3389/fonc.2013.00085
Xu Z, Robitaille AM, Berndt JD, Davidson KC, Fischer KA, Mathieu J et al (2016) Wnt/beta-catenin signaling promotes self-renewal and inhibits the primed state transition in naive human embryonic stem cells. Proc Natl Acad Sci 113(42):E6382–E6390. https://doi.org/10.1073/pnas.1613849113
Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ et al (2011) MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 334(6053):252–255
Kampjarvi K, Park MJ, Mehine M, Kim NH, Clark AD, Butzow R et al (2014) Mutations in exon 1 highlight the role of MED12 in uterine leiomyomas. Hum Mutat 35(9):1136–1141. https://doi.org/10.1002/humu.22612
Kim S, Xu X, Hecht A, Boyer TG (2006) Mediator is a transducer of Wnt/β-catenin signaling. J Biol Chem 281(20):14066–14075
Rocha PP, Scholze M, Bleiß W, Schrewe H (2010) Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development 137(16):2723–2731
El Andaloussi A, Al-Hendy A, Ismail N, Boyer TG, Halder SK (2020) Introduction of somatic mutation in MED12 induces Wnt4/beta-catenin and disrupts autophagy in human uterine myometrial cell. Reprod Sci 27(3):823–832. https://doi.org/10.1007/s43032-019-00084-7
Corachan A, Trejo MG, Carbajo-Garcia MC, Monleon J, Escrig J, Faus A et al (2020) Vitamin D as an effective treatment in human uterine leiomyomas independent of mediator complex subunit 12 mutation. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2020.07.049
Mehine M, Kaasinen E, Heinonen HR, Makinen N, Kampjarvi K, Sarvilinna N et al (2016) Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci 113(5):1315–1320. https://doi.org/10.1073/pnas.1518752113
Wozniak MA, Chen CS (2009) Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10(1):34–43. https://doi.org/10.1038/nrm2592
Hoffman BD, Grashoff C, Schwartz MA (2011) Dynamic molecular processes mediate cellular mechanotransduction. Nature 475(7356):316–323. https://doi.org/10.1038/nature10316
Gillespie PG, Walker RG (2001) Molecular basis of mechanosensory transduction. Nature 413(6852):194–202. https://doi.org/10.1038/35093011
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260(5111):1124–1127
Broders-Bondon F, Nguyen Ho-Bouldoires TH, Fernandez-Sanchez ME, Farge E (2018) Mechanotransduction in tumor progression: the dark side of the force. J Cell Biol 217(5):1571–1587. https://doi.org/10.1083/jcb.201701039
Zhang J, Zhou Y, Tang PMK, Cheng ASL, Yu J, To KF et al (2019) Mechanotransduction and cytoskeleton remodeling shaping yap1 in gastric tumorigenesis. Int J Mol Sci 20(7):1576. https://doi.org/10.3390/ijms20071576
Mammoto T, Ingber DE (2010) Mechanical control of tissue and organ development. Development 137(9):1407–1420. https://doi.org/10.1242/dev.024166
Pukhlyakova E, Aman AJ, Elsayad K, Technau U (2018) β-Catenin–dependent mechanotransduction dates back to the common ancestor of Cnidaria and Bilateria. Proc Natl Acad Sci 115(24):6231–6236
Brunet T, Bouclet A, Ahmadi P, Mitrossilis D, Driquez B, Brunet AC et al (2013) Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria. Nat Commun 4:2821. https://doi.org/10.1038/ncomms3821
Warboys CM (2018) Mechanoactivation of Wnt/β-catenin pathways in health and disease. Emerg Top Life Scis 2(5):701–712
Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P et al (2011) Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19(6):776–791
Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I et al (2014) Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med 20(4):360–367. https://doi.org/10.1038/nm.3497
Benham-Pyle BW, Pruitt BL, Nelson WJ (2015) Mechanical strain induces E-cadherin–dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 348(6238):1024–1027
Röper JC, Mitrossilis D, Stirnemann G, Waharte F, Brito I, Fernandez-Sanchez ME et al (2018) The major β-catenin/E-cadherin junctional binding site is a primary molecular mechano-transductor of differentiation in vivo. Elife 7:e33381
Fernandez-Sanchez ME, Barbier S, Whitehead J, Béalle G, Michel A, Latorre-Ossa H et al (2015) Mechanical induction of the tumorigenic β-catenin pathway by tumour growth pressure. Nature 523(7558):92–95
Du J, Zu Y, Li J, Du S, Xu Y, Zhang L et al (2016) Extracellular matrix stiffness dictates Wnt expression through integrin pathway. Sci Rep 6:20395
Malik M, Segars J, Catherino WH (2012) Integrin beta1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol 31(7–8):389–397. https://doi.org/10.1016/j.matbio.2012.09.005
Leppert PC, Catherino WH, Segars JH (2006) A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol 195(2):415–420
Baarsma HA, Menzen MH, Halayko AJ, Meurs H, Kerstjens HA, Gosens R (2011) β-Catenin signaling is required for TGF-β1-induced extracellular matrix production by airway smooth muscle cells. Am J Physiol-Lung Cell Mol Physiol 301(6):L956–L965
Arici A, Sozen I (2000) Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 73(5):1006–1011. https://doi.org/10.1016/s0015-0282(00)00418-0
Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK et al (2004) Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosome Cancer 40(3):204–217
Fuentes N, Silveyra P (2019) Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol 116:135
Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A (2017) Estrogen receptors and signaling in fibroids: role in pathobiology and therapeutic implications. Reprod Sci 24(9):1235–1244. https://doi.org/10.1177/1933719116678686
Sant’Anna GDS, Brum IS, Branchini G, Pizzolato LS, Capp E, Corleta HVE (2017) Ovarian steroid hormones modulate the expression of progesterone receptors and histone acetylation patterns in uterine leiomyoma cells. Gynecol Endocrinol 33(8):629–633. https://doi.org/10.1080/09513590.2017.1301924
Hou X, Tan Y, Li M, Dey SK, Das SK (2004) Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18(12):3035–3049
Zhang L, Xiong W, Xiong Y, Liu H, Liu Y (2016) 17 β-Estradiol promotes vascular endothelial growth factor expression via the Wnt/β-catenin pathway during the pathogenesis of endometriosis. Mol. Hum. Reprod 22(7):526–535. https://doi.org/10.1093/molehr/gaw025
Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T (2010) Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151(6):2433–2442
Lange CA (2008) Integration of progesterone receptor action with rapid signaling events in breast cancer models. J Steroid Biochem Mol Biol 108(3–5):203–212. https://doi.org/10.1016/j.jsbmb.2007.09.019
Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL et al (2001) Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8(2):269–280
Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK et al (2000) Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14(6):650–654
Rider V, Talbott A, Bhusri A, Krumsick Z, Foster S, Wormington J et al (2016) WINGLESS (WNT) signaling is a progesterone target for rat uterine stromal cell proliferation. J Endocrinol 229(2):197–207. https://doi.org/10.1530/JOE-15-0523
Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC (2006) Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 91(4):1453–1461. https://doi.org/10.1210/jc.2005-0769
Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D et al (2011) WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25(4):1176–1187
Massague J (2012) TGFbeta signalling in context. Nat Rev Mol Cell Biol 13(10):616–630. https://doi.org/10.1038/nrm3434
Derynck R, Budi EH (2019) Specificity, versatility, and control of TGF-beta family signaling. Sci Signal 12(570):eaav5183. https://doi.org/10.1126/scisignal.aav5183
Lee BS, Nowak RA (2001) Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-beta 3 (TGF beta 3) and altered responses to the antiproliferative effects of TGF beta. J Clin Endocrinol Metab 86(2):913–920
Joseph DS, Malik M, Nurudeen S, Catherino WH (2010) Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril 93(5):1500–1508. https://doi.org/10.1016/j.fertnstert.2009.01.081
Attisano L, Labbe E (2004) TGFbeta and Wnt pathway cross-talk. Cancer Metastasis Rev 23(1–2):53–61. https://doi.org/10.1023/a:1025811012690
Yu JS, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143(17):3050–3060. https://doi.org/10.1242/dev.137075
Hoekstra AV, Sefton EC, Berry E, Lu Z, Hardt J, Marsh E et al (2009) Progestins activate the AKT pathway in leiomyoma cells and promote survival. J Clin Endocrinol Metab 94(5):1768–1774. https://doi.org/10.1210/jc.2008-2093
Karra L, Shushan A, Ben-Meir A, Rojansky N, Klein BY, Shveiky D et al (2010) Changes related to phosphatidylinositol 3-kinase/Akt signaling in leiomyomas: possible involvement of glycogen synthase kinase 3α and cyclin D2 in the pathophysiology. Fertil Steril 93(8):2646–2651
Sefton EC, Qiang W, Serna V, Kurita T, Wei JJ, Chakravarti D et al (2013) MK-2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth. Endocrinology 154(11):4046–4057. https://doi.org/10.1210/en.2013-1389
Xu X, Lu Z, Qiang W, Vidimar V, Kong B, Kim JJ et al (2014) Inactivation of AKT induces cellular senescence in uterine leiomyoma. Endocrinology 155(4):1510–1519. https://doi.org/10.1210/en.2013-1929
Vidimar V, Chakravarti D, Bulun SE, Yin P, Nowak R, Wei JJ et al (2018) The AKT/BCL-2 axis mediates survival of uterine leiomyoma in a novel 3d spheroid model. Endocrinology 159(3):1453–1462. https://doi.org/10.1210/en.2017-03191
Xie J, Ubango J, Ban Y, Chakravarti D, Kim JJ, Wei JJ (2018) Comparative analysis of AKT and the related biomarkers in uterine leiomyomas with MED12, HMGA2, and FH mutations. Genes Chromosomes Cancer 57(10):485–494. https://doi.org/10.1002/gcc.22643
Crabtree JS, Jelinsky SA, Harris HA, Choe SE, Cotreau MM, Kimberland ML et al (2009) Comparison of human and rat uterine leiomyomata: identification of a dysregulated mammalian target of rapamycin pathway. Cancer Res 69(15):6171–6178
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H et al (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282(15):11221–11229. https://doi.org/10.1074/jbc.M611871200
Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH et al (2001) Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem 276(20):17479–17483. https://doi.org/10.1074/jbc.C000880200
Al-Hendy A, Diamond MP, Boyer TG, Halder SK (2016) Vitamin D3 inhibits Wnt/β-catenin and mTOR signaling pathways in human uterine fibroid cells. J Clin Endocrinol Metab 101(4):1542–1551
Bashanfer SAAS, Saleem M, Heidenreich O, Moses EJ, Yusoff NM (2019) Disruption of MAPK1 expression in the ERK signalling pathway and the RUNX1-RUNX1T1 fusion gene attenuate the differentiation and proliferation and induces the growth arrest in t (8; 21) leukaemia cells. Oncol Rep 41(3):2027–2040
Kurtzeborn K, Kwon HN, Kuure S (2019) MAPK/ERK signaling in regulation of renal differentiation. Int J Mol Sci. https://doi.org/10.3390/ijms20071779
Kolch W (2000) Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J 351(Pt 2):289–305
Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26(22):3291–3310. https://doi.org/10.1038/sj.onc.1210422
Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE et al (2008) Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med 14(5–6):264–275. https://doi.org/10.2119/2007-00101.Yu
Nierth-Simpson EN, Martin MM, Chiang T-C, Melnik LI, Rhodes LV, Muir SE et al (2009) Human uterine smooth muscle and leiomyoma cells differ in their rapid 17β-estradiol signaling: implications for proliferation. Endocrinology 150(5):2436–2445
Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY (2005) Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci 118(Pt 2):313–322. https://doi.org/10.1242/jcs.01601
Jeong WJ, Yoon J, Park JC, Lee SH, Lee SH, Kaduwal S et al (2012) Ras stabilization through aberrant activation of Wnt/beta-catenin signaling promotes intestinal tumorigenesis. Sci Signal 5(219):ra30. https://doi.org/10.1126/scisignal.2002242
Peng L, Wen Y, Han Y, Wei A, Shi G, Mizuguchi M et al (2009) Expression of insulin-like growth factors (IGFs) and IGF signaling: molecular complexity in uterine leiomyomas. Fertil Steril 91(6):2664–2675
Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T (2001) Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab 86(11):5593–5599. https://doi.org/10.1210/jcem.86.11.8008
Gkioka E, Msaouel P, Philippou A, Vlaghogiannis NI, Vogkou CT, Margiolis A et al (2015) Review: the role of insulin-like growth factor-1 signaling pathways in uterine leiomyoma. Vivo 29(6):637–649
Moravek MB, Yin P, Coon JS, Ono M, Druschitz SA, Malpani SS et al (2017) Paracrine pathways in uterine leiomyoma stem cells involve insulinlike growth factor 2 and insulin receptor A. J Clin Endocrinol Metab 102(5):1588–95. https://doi.org/10.1210/jc.2016-3497
Schlupf J, Steinbeisser H (2014) IGF antagonizes the Wnt/β-Catenin pathway and promotes differentiation of extra-embryonic endoderm. Differentiation 87(5):209–219
Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C et al (2001) Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 20(2):252–259. https://doi.org/10.1038/sj.onc.1204064
Zhao B, Tumaneng K, Guan KL (2011) The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 13(8):877–883. https://doi.org/10.1038/ncb2303
Pan D (2010) The hippo signaling pathway in development and cancer. Dev Cell 19(4):491–505. https://doi.org/10.1016/j.devcel.2010.09.011
Islam MS, Maher JY, Afrin S, Su S-C, Segars J (2019) Verteporfin inhibits fibrosis, inflammation and angiogenesis related genes in uterine fibroid cells. Fertil Steril 112(3):e349
Piersma B, Bank RA, Boersema M (2015) Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front Med 2:59
Luo K (2017) Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol 9(1):a022137
Kim M, Jho Eh (2014) Cross-talk between Wnt/β-catenin and Hippo signaling pathways: a brief review. BMB reports 47(10):540. https://doi.org/10.5483/BMBRep.2014.47.10.177
Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA et al (2010) The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell 18(4):579–591. https://doi.org/10.1016/j.devcel.2010.03.007
Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S et al (2012) Role of TAZ as mediator of Wnt signaling. Cell 151(7):1443–1456
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S et al (2014) YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158(1):157–170. https://doi.org/10.1016/j.cell.2014.06.013
Egan SE, St-Pierre B, Leow CC (1998) Notch receptors, partners and regulators: from conserved domains to powerful functions. Curr Top Microbiol Immunol 228:273–324. https://doi.org/10.1007/978-3-642-80481-6_11
Zlobin A, Bloodworth JC, Baker AT, Osipo C (2019) Notch signaling pathway in carcinogenesis. In: Badve S, Kumar GL (eds) Predictive biomarkers in oncology: applications in precision medicine. Springer International Publishing, Cham, pp 223–230
McIntyre B, Asahara T, Alev C (2020) Overview of basic mechanisms of notch signaling in development and disease. In: Reichrath J, Reichrath S (eds) Notch signaling in embryology and cancer: molecular biology of notch signaling. Springer International Publishing, Cham, pp 9–27
Kiyokawa H, Morimoto M (2020) Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Dev Growth Differ 62(1):67–79. https://doi.org/10.1111/dgd.12628
Kavian N, Servettaz A, Weill B, Batteux F (2012) Suppl 1: new insights into the mechanism of notch signalling in fibrosis. Open Rheumatol J 6:96
Gonzalez-Foruria I, Santulli P, Chouzenoux S, Carmona F, Chapron C, Batteux F (2017) Dysregulation of the ADAM17/Notch signalling pathways in endometriosis: from oxidative stress to fibrosis. Mol Hum Reprod 23(7):488–499. https://doi.org/10.1093/molehr/gax028
Collu GM, Hidalgo-Sastre A, Brennan K (2014) Wnt-Notch signalling crosstalk in development and disease. Cell Mol Life Sci 71(18):3553–3567. https://doi.org/10.1007/s00018-014-1644-x
Collu GM, Hidalgo-Sastre A, Acar A, Bayston L, Gildea C, Leverentz MK et al (2012) Dishevelled limits Notch signalling through inhibition of CSL. Development 139(23):4405–4415. https://doi.org/10.1242/dev.081885
Kwon C, Cheng P, King IN, Andersen P, Shenje L, Nigam V et al (2011) Notch post-translationally regulates beta-catenin protein in stem and progenitor cells. Nat Cell Biol 13(10):1244–1251. https://doi.org/10.1038/ncb2313
Liu XH, Wu Y, Yao S, Levine AC, Kirschenbaum A, Collier L et al (2013) Androgens up-regulate transcription of the Notch inhibitor Numb in C2C12 myoblasts via Wnt/beta-catenin signaling to T cell factor elements in the Numb promoter. J Biol Chem 288(25):17990–17998. https://doi.org/10.1074/jbc.M113.478487
Christman GM, Tang H, Ahmad I, Stribley JM (2007) Differential expression of the Notch signal transduction pathway: ligands, receptors and Numb in uterine leiomyomas vs myometrium. Fertil Steril 88:572. https://doi.org/10.1016/j.fertnstert.2007.07.242
Ciebiera M, Lukaszuk K, Meczekalski B, Ciebiera M, Wojtyla C, Slabuszewska-Jozwiak A et al (2017) Alternative oral agents in prophylaxis and therapy of uterine fibroids-an up-to-date review. Int J Mol Sci 18(12):2586. https://doi.org/10.3390/ijms18122586
Arioka M, Igawa K, Tomooka K, Nakatsu Y, Tsuzuki T, Nakabeppu Y, Kitazono T, Sasaguri T (2017) Celecoxib and 2,5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/β-catenin signaling pathway. Cancer Science 108(1):108–115. https://doi.org/10.1111/cas.13106
Egashira I, Takahashi-Yanaga F, Nishida R, Arioka M, Igawa K, Tomooka K et al (2017) Celecoxib and 2, 5-dimethylcelecoxib inhibit intestinal cancer growth by suppressing the Wnt/β-catenin signaling pathway. Cancer Sci 108(1):108–115
Sareddy GR, Kesanakurti D, Kirti PB, Babu PP (2013) Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/β-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem Res 38(11):2313–2322. https://doi.org/10.1007/s11064-013-1142-9
Pendas-Franco N, Aguilera O, Pereira F, González-Sancho JM, Munoz A (2008) Vitamin D and Wnt/β-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res 28(5A):2613–2623
Amado NG, Predes D, Fonseca BF, Cerqueira DM, Reis AH, Dudenhoeffer AC et al (2014) Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J Biol Chem 289(51):35456–35467. https://doi.org/10.1074/jbc.M114.621599
Lazarian G, Friedrich C, Quinquenel A, Tran J, Ouriemmi S, Dondi E et al (2020) Stabilization of β-catenin upon B-cell receptor signaling promotes NF-kB target genes transcription in mantle cell lymphoma. Oncogene 39(14):2934–2947
Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA et al (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461(7264):614–620. https://doi.org/10.1038/nature08356
James RG, Davidson KC, Bosch KA, Biechele TL, Robin NC, Taylor RJ et al (2012) WIKI4, a novel inhibitor of tankyrase and Wnt/ß-catenin signaling. PloS one 7(12):e50457. https://doi.org/10.1371/journal.pone.0050457
Waaler J, Machon O, Tumova L, Dinh H, Korinek V, Wilson SR et al (2012) A novel tankyrase inhibitor decreases canonical wnt signaling in colon carcinoma cells and reduces tumor growth in conditional apc mutant mice. Cancer Res 72(11):2822–2832. https://doi.org/10.1158/0008-5472.can-11-3336
Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J et al (2016) Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene 35(17):2197–2207. https://doi.org/10.1038/onc.2015.280
Cheng D, Liu J, Han D, Zhang G, Gao W, Hsieh MH et al (2016) Discovery of pyridinyl acetamide derivatives as potent, selective, and orally bioavailable porcupine inhibitors. ACS Med Chem Lett 7(7):676–680
Kothari V, Goodwin JF, Zhao SG, Drake JM, Yin Y, Chang SL et al (2019) DNA-dependent protein kinase drives prostate cancer progression through transcriptional regulation of the wnt signaling pathway. Clin Cancer Res 25(18):5608–5622
Wellenstein MD, Coffelt SB, Duits DEM, van Miltenburg MH, Slagter M, de Rink I et al (2019) Loss of p53 triggers WNT-dependent systemic inflammation to drive breast cancer metastasis. Nature 572(7770):538–542. https://doi.org/10.1038/s41586-019-1450-6
Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA et al (2013) Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res 73(2):502–507. https://doi.org/10.1158/0008-5472.CAN-12-2258
Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N et al (2013) Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci 110(31):12649–12654. https://doi.org/10.1073/pnas.1307218110
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T et al (2013) Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci 110(50):20224–20229. https://doi.org/10.1073/pnas.1314239110
You L, Zhang C, Yarravarapu N, Morlock L, Wang X, Zhang L et al (2016) Development of a triazole class of highly potent Porcn inhibitors. Bioorg Med Chem Lett 26(24):5891–5895. https://doi.org/10.1016/j.bmcl.2016.11.012
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W et al (2009) Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5(2):100–107. https://doi.org/10.1038/nchembio.137
Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M et al (2004) A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci 101(34):12682–12687. https://doi.org/10.1073/pnas.0404875101
Waaler J, Machon O, von Kries JP, Wilson SR, Lundenes E, Wedlich D et al (2011) Novel synthetic antagonists of canonical wnt signaling inhibit colorectal cancer cell growth. Cancer Res 71(1):197–205. https://doi.org/10.1158/0008-5472.can-10-1282
Cha PH, Cho YH, Lee SK, Lee J, Jeong WJ, Moon BS et al (2016) Small-molecule binding of the axin RGS domain promotes β-catenin and Ras degradation. Nat Chem Biol 12(8):593–600. https://doi.org/10.1038/nchembio.2103
Cook J, Walker C (2004) The Eker rat: establishing a genetic paradigm linking renal cell carcinoma and uterine leiomyoma. Curr Mol Med 4(8):813–824
Sheng R, Kim H, Lee H, Xin Y, Chen Y, Tian W et al (2014) Cholesterol selectively activates canonical Wnt signalling over non-canonical Wnt signalling. Nat Commun 5:4393. https://doi.org/10.1038/ncomms5393
Wong HC, Mao J, Nguyen JT, Srinivas S, Zhang W, Liu B et al (2000) Structural basis of the recognition of the dishevelled DEP domain in the Wnt signaling pathway. Nat Struct Biol 7(12):1178–1184. https://doi.org/10.1038/82047
Tauriello DV, Jordens I, Kirchner K, Slootstra JW, Kruitwagen T, Bouwman BA et al (2012) Wnt/beta-catenin signaling requires interaction of the dishevelled DEP domain and C terminus with a discontinuous motif in Frizzled. Proc Natl Acad Sci 109(14):E812–E820. https://doi.org/10.1073/pnas.1114802109
Miyakoshi T, Kajiya H, Miyajima K, Takei M, Tobita M, Takekoshi S et al (2009) The expression of wnt4 is regulated by estrogen via an estrogen receptor alpha-dependent pathway in rat pituitary growth hormone-producing cells. Acta Histochem Cytochem 42(6):205–213. https://doi.org/10.1267/ahc.09033
Malik M, Britten J, Borahay M, Segars J, Catherino WH (2018) Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production. Fertil Steril 110(7):1398–407.e1. https://doi.org/10.1016/j.fertnstert.2018.07.024
Malik M, Catherino W, Laknaur A, Ali M, Al-Hendy A, Segars J et al (2017) Synergistic effects of simvastatin and ulipristal acetate on uterine leiomyoma. Fertil Steril 108(3):e65
Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A et al (2015) Novel effects of simvastatin on uterine fibroid tumors: in vitro and patient-derived xenograft mouse model study. Am J Obstet Gynecol 213(2):196.e1-196.e8. https://doi.org/10.1016/j.ajog.2015.03.055
Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GD, Al-Hendy A et al (2014) Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J Biol Chem 289(51):35075–35086. https://doi.org/10.1074/jbc.M114.583575
Afrin S, Islam MS, Patzkowsky K, Malik M, Catherino WH, Segars JH et al (2020) Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2020.05.012
Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N (1996) The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in wingless processing. Genes Dev 10(24):3116–3128. https://doi.org/10.1101/gad.10.24.3116
Kim YM, Kahn M (2014) The role of the Wnt signaling pathway in cancer stem cells: prospects for drug development. Res Rep Biochem 4:1–12. https://doi.org/10.2147/RRBC.S53823
Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T et al (2013) Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci 110(50):20224–20229
Mullighan CG, Zhang J, Kasper LH, Lerach S, Payne-Turner D, Phillips LA et al (2011) CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 471(7337):235–239. https://doi.org/10.1038/nature09727
Wang X, Moon J, Dodge ME, Pan X, Zhang L, Hanson JM et al (2013) The development of highly potent inhibitors for porcupine. J Med Chem 56(6):2700–2704. https://doi.org/10.1021/jm400159c
Fischer MM, Cancilla B, Yeung VP, Cattaruzza F, Chartier C, Murriel CL et al (2017) WNT antagonists exhibit unique combinatorial antitumor activity with taxanes by potentiating mitotic cell death. Sci Adv 3(6):e1700090. https://doi.org/10.1126/sciadv.1700090
Jimeno A, Gordon M, Chugh R, Messersmith W, Mendelson D, Dupont J et al (2017) A first-in-human phase i study of the anticancer stem cell agent ipafricept (OMP-54f28), a decoy receptor for wnt ligands, in patients with advanced solid tumors. Clin Cancer Res 23(24):7490–7497. https://doi.org/10.1158/1078-0432.CCR-17-2157
Moore KN, Gunderson CC, Sabbatini P, McMeekin DS, Mantia-Smaldone G, Burger RA et al (2019) A phase 1b dose escalation study of ipafricept (OMP54F28) in combination with paclitaxel and carboplatin in patients with recurrent platinum-sensitive ovarian cancer. Gynecol Oncol 154(2):294–301. https://doi.org/10.1016/j.ygyno.2019.04.001
Giraudet AL, Cassier PA, Iwao-Fukukawa C, Garin G, Badel JN, Kryza D et al (2018) A first-in-human study investigating biodistribution, safety and recommended dose of a new radiolabeled MAb targeting FZD10 in metastatic synovial sarcoma patients. BMC Cancer 18(1):646. https://doi.org/10.1186/s12885-018-4544-x
Takemaru KI, Moon RT (2000) The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol 149(2):249–254. https://doi.org/10.1083/jcb.149.2.249
Lee J-H et al (2020) Phase 1 study of CWP232291 in patients with relapsed or refractory acute myeloid leukemia and myelodysplastic syndrome. Blood Adv 4(9):2032–2043. https://doi.org/10.1182/bloodadvances.2019000757
Manasanch EE, Yoon SS, Min CK, Kim JS, Shain KH, Hauptschein R et al (2017) Interim results from the phase 1a/1b dose-finding study of CWP232291 (CWP291) in relapsed or refractory myeloma (RRMM) alone or in combination with lenalidomide and dexamethasone. Blood 130(Supplement 1):3091
Yoon S-S, Min C-K, Kim JS, Manasanch EE, Hauptschein R, Choi J et al (2016) Ongoing phase 1a/1b dose-finding study of CWP232291 (CWP291) in relapsed or refractory multiple myeloma (MM). American Society of Hematology , Washington DC
Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H et al (2014) Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159(1):80–93. https://doi.org/10.1016/j.cell.2014.08.007
Larriba MJ, Gonzalez-Sancho JM, Barbachano A, Niell N, Ferrer-Mayorga G, Munoz A (2013) Vitamin D Is a multilevel repressor of wnt/b-catenin signaling in cancer cells. Cancers 5(4):1242–1260. https://doi.org/10.3390/cancers5041242
Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK et al (2008) Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer 122(2):267–273. https://doi.org/10.1002/ijc.23097
Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I et al (2005) Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res 11(18):6738–6744. https://doi.org/10.1158/1078-0432.CCR-05-0171
Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS et al (2010) Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res 70(19):7392–7399. https://doi.org/10.1158/0008-5472.CAN-10-2027
Holcombe RF, Martinez M, Planutis K, Planutiene M (2015) Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr J 14(1):62
Dietrich L, Rathmer B, Ewan K, Bange T, Heinrichs S, Dale TC et al (2017) Cell permeable stapled peptide inhibitor of wnt signaling that targets x3b2;-catenin protein-protein interactions. Cell Chem Biol 24(8):958–68.e5. https://doi.org/10.1016/j.chembiol.2017.06.013
Gonsalves FC, Klein K, Carson BB, Katz S, Ekas LA, Evans S et al (2011) An RNAi-based chemical genetic screen identifies three small-molecule inhibitors of the Wnt/wingless signaling pathway. Proc Natl Acad Sci 108(15):5954–5963. https://doi.org/10.1073/pnas.1017496108
Leal LF, Bueno AC, Gomes DC, Abduch R, de Castro M, Antonini SR (2015) Inhibition of the Tcf/beta-catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget 6(40):43016–43032. https://doi.org/10.18632/oncotarget.5513
Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI et al (2016) A small-molecule antagonist of the β-catenin/TCF4 interaction blocks the self-renewal of cancer stem cells and suppresses tumorigenesis. Cancer Res 76(4):891–901. https://doi.org/10.1158/0008-5472.can-15-1519
Gedaly R, Galuppo R, Daily MF, Shah M, Maynard E, Chen C et al (2014) Targeting the Wnt/β-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PloS one 9(6):e99272. https://doi.org/10.1371/journal.pone.0099272
Masuda M, Uno Y, Ohbayashi N, Ohata H, Mimata A, Kukimoto-Niino M et al (2016) TNIK inhibition abrogates colorectal cancer stemness. Nat Commun 7(1):12586. https://doi.org/10.1038/ncomms12586
Ewan K, Pajak B, Stubbs M, Todd H, Barbeau O, Quevedo C et al (2010) A useful approach to identify novel small-molecule inhibitors of Wnt-dependent transcription. Cancer Res 70(14):5963–5973. https://doi.org/10.1158/0008-5472.can-10-1028
Funding
This work was supported, in part, by NIH Grant 1R01HD094380 to Mostafa Borahay.
Author information
Authors and Affiliations
Contributions
ME performed literature review, manuscript drafting, and critical discussion; SKS and MAB contributed to study design, literature review, manuscript drafting, and critical discussion; SA and MSI performed manuscript drafting and critical discussion.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El Sabeh, M., Saha, S.K., Afrin, S. et al. Wnt/β-catenin signaling pathway in uterine leiomyoma: role in tumor biology and targeting opportunities. Mol Cell Biochem 476, 3513–3536 (2021). https://doi.org/10.1007/s11010-021-04174-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-021-04174-6