Abstract
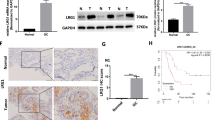
Renal cell carcinoma (RCC) is a kind of malignant tumor with high recurrence, and it is urgent to find molecular markers for diagnosis and prognosis of RCC. Our study investigated the expression and function of integrin αMβ2 in RCC cells, aiming to understand the role of integrin αMβ2 in RCC and develop new therapeutic target for RCC. Overexpression and knockdown of lymphoid enhancer-binding factor 1 (LEF1) were performed using vector containing full-length cDNA and via siRNA technology, respectively. The expressions of mRNA and protein were detected by RT-PCR and Western blot, respectively. Proliferation of RCC cell was analyzed using WST-1 assay, and metastasis of RCC cell was evaluated using the transwell system. Our results demonstrated that LEF1 and integrin αMβ2 were up-regulated in RCC cells via TGF-β1-dependent mechanism, and LEF1 together with β-catenin directly increased integrin αMβ2 level. On the other hand, TGF-β1-induced proliferation, migration and invasion were suppressed by function-blocking antibody against integrin αMβ2 in RCC cells. In addition, integrin αMβ2 is crucial for LEF1 mediated cell invasion by regulating MMP-2, MMP-9 and calpain-2 secretion in RCC cells. LEF1/integrin αMβ2 expression was regulated by TGF-β1, and LEF1/integrin αMβ2 was involved in TGF-β1’s improvement effects on the proliferation and metastasis of RCC. Blocking integrin αMβ2 activity could be a therapeutic option for patients with advanced RCC.






Similar content being viewed by others
References
Porta C, Paglino C, Grunwald V (2014) Sunitinib re-challenge in advanced renal-cell carcinoma. Br J Cancer 111(6):1047–1053. https://doi.org/10.1038/bjc.2014.214
Chiong E, Tay MH, Tan MH, Kumar S, Sim HG, Teh BT, Umbas R, Chau NM (2012) Management of kidney cancer in Asia: resource-stratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol 13(11):e482–491. https://doi.org/10.1016/S1470-2045(12)70433-3
Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, De La Taille A, Tostain J, Artibani W, Abbou CC, Lobel B, Guille F, Chopin DK, Mulders PF, Wood CG, Swanson DA, Figlin RA, Belldegrun AS, Pantuck AJ (2005) Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol 23(12):2763–2771. https://doi.org/10.1200/JCO.2005.07.055
Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA (2009) Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 27(22):3584–3590. https://doi.org/10.1200/JCO.2008.20.1293
Wu D, Xu Y, Ding T, Zu Y, Yang C, Yu L (2017) Pairing of integrins with ECM proteins determines migrasome formation. Cell Res 27(11):1397–1400. https://doi.org/10.1038/cr.2017.108
Barczyk M, Carracedo S, Gullberg D (2010) Integrins. Cell Tissue Res 339(1):269–280. https://doi.org/10.1007/s00441-009-0834-6
Rucci N, Teti A (2016) The “love-hate” relationship between osteoclasts and bone matrix. Matrix Biol 52–54:176–190. https://doi.org/10.1016/j.matbio.2016.02.009
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687. https://doi.org/10.1016/s0092-8674(02)00971-6
Streuli CH (2009) Integrins and cell-fate determination. J Cell Sci 122(Pt 2):171–177. https://doi.org/10.1242/jcs.018945
Abram CL, Lowell CA (2009) The ins and outs of leukocyte integrin signaling. Annu Rev Immunol 27:339–362. https://doi.org/10.1146/annurev.immunol.021908.132554
Prieto J, Eklund A, Patarroyo M (1994) Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol 156(1):191–211. https://doi.org/10.1006/cimm.1994.1164
Shang XZ, Issekutz AC (1997) Beta 2 (CD18) and beta 1 (CD29) integrin mechanisms in migration of human polymorphonuclear leucocytes and monocytes through lung fibroblast barriers: shared and distinct mechanisms. Immunology 92(4):527–535. https://doi.org/10.1046/j.1365-2567.1997.00372.x
Graff JC, Jutila MA (2007) Differential regulation of CD11b on gammadelta T cells and monocytes in response to unripe apple polyphenols. J Leukoc Biol 82(3):603–607. https://doi.org/10.1189/jlb.0207125
Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, Sharma A, Kaplan D, Greaves DR, Wang Y, Simon DI (2008) Down-regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood 112(12):4699–4711. https://doi.org/10.1182/blood-2008-01-137018
Shi C, Zhang X, Chen Z, Sulaiman K, Feinberg MW, Ballantyne CM, Jain MK, Simon DI (2004) Integrin engagement regulates monocyte differentiation through the forkhead transcription factor Foxp1. J Clin Investig 114(3):408–418. https://doi.org/10.1172/JCI21100
Xue ZH, Zhao CQ, Chua GL, Tan SW, Tang XY, Wong SC, Tan SM (2010) Integrin alphaMbeta2 clustering triggers phosphorylation and activation of protein kinase C delta that regulates transcription factor Foxp1 expression in monocytes. J Immunol 184(7):3697–3709. https://doi.org/10.4049/jimmunol.0903316
Varga G, Balkow S, Wild MK, Stadtbaeumer A, Krummen M, Rothoeft T, Higuchi T, Beissert S, Wethmar K, Scharffetter-Kochanek K, Vestweber D, Grabbe S (2007) Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood 109(2):661–669. https://doi.org/10.1182/blood-2005-12-023044
Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, Bhardwaj N (2006) The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood 108(3):947–955. https://doi.org/10.1182/blood-2005-12-4812
Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T (2002) The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med 196(5):679–691. https://doi.org/10.1084/jem.20020267
Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA (2000) Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J Exp Med 192(2):193–204. https://doi.org/10.1084/jem.192.2.193
Waterman ML, Fischer WH, Jones KA (1991) A thymus-specific member of the HMG protein family regulates the human T cell receptor C alpha enhancer. Genes Dev 5(4):656–669. https://doi.org/10.1101/gad.5.4.656
Travis A, Amsterdam A, Belanger C, Grosschedl R (1991) LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev 5(5):880–894. https://doi.org/10.1101/gad.5.5.880
Liu LJ, Yu JJ, Xu XL (2017) MicroRNA-93 inhibits apoptosis and promotes proliferation, invasion and migration of renal cell carcinoma ACHN cells via the TGF-beta/Smad signaling pathway by targeting RUNX3. Am J Transl Res 9(7):3499–3513
Sjolund J, Bostrom AK, Lindgren D, Manna S, Moustakas A, Ljungberg B, Johansson M, Fredlund E, Axelson H (2011) The notch and TGF-beta signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS ONE 6(8):e23057. https://doi.org/10.1371/journal.pone.0023057
Bostrom AK, Lindgren D, Johansson ME, Axelson H (2013) Effects of TGF-beta signaling in clear cell renal cell carcinoma cells. Biochem Biophys Res Commun 435(1):126–133. https://doi.org/10.1016/j.bbrc.2013.04.054
Huang F, Newman E, Theodorescu D, Kerbel RS, Friedman E (1995) Transforming growth factor beta 1 (TGF beta 1) is an autocrine positive regulator of colon carcinoma U9 cells in vivo as shown by transfection of a TGF beta 1 antisense expression plasmid. Cell Growth Differ 6(12):1635–1642
Wang J, Chen J, Zhang K, Zhao Y, Nor JE, Wu J (2011) TGF-beta1 regulates the invasive and metastatic potential of mucoepidermoid carcinoma cells. J Oral Pathol Med 40(10):762–768. https://doi.org/10.1111/j.1600-0714.2011.01051.x
Ananth S, Knebelmann B, Gruning W, Dhanabal M, Walz G, Stillman IE, Sukhatme VP (1999) Transforming growth factor beta1 is a target for the von Hippel-Lindau tumor suppressor and a critical growth factor for clear cell renal carcinoma. Cancer Res 59(9):2210–2216
Lebdai S, Verhoest G, Parikh H, Jacquet SF, Bensalah K, Chautard D, Rioux-Leclercq N, Azzouzi AR, Bigot P (2015) Identification and validation of TGFBI as a promising prognosis marker of clear cell renal cell carcinoma. Urol Oncol 33(2):69.e11–69.e68. https://doi.org/10.1016/j.urolonc.2014.06.005
Huang W, Cen S, Kang XL, Wang WF, Wang Y, Chen X (2016) TGF-beta1-induced Fascin1 promotes cell invasion and metastasis of human 786-0 renal carcinoma cells. Acta Histochem 118(2):144–151. https://doi.org/10.1016/j.acthis.2015.12.005
Edlund S, Lee SY, Grimsby S, Zhang S, Aspenstrom P, Heldin CH, Landstrom M (2005) Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol 25(4):1475–1488. https://doi.org/10.1128/MCB.25.4.1475-1488.2005
Feldkoren B, Hutchinson R, Rapoport Y, Mahajan A, Margulis V (2017) Integrin signaling potentiates transforming growth factor-beta 1 (TGF-beta1) dependent down-regulation of E-Cadherin expression—important implications for epithelial to mesenchymal transition (EMT) in renal cell carcinoma. Exp Cell Res 355(2):57–66. https://doi.org/10.1016/j.yexcr.2017.03.051
Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM, Tang MJ (2010) Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol 177(4):1743–1754. https://doi.org/10.2353/ajpath.2010.091183
Li TW, Ting JH, Yokoyama NN, Bernstein A, van de Wetering M, Waterman ML (2006) Wnt activation and alternative promoter repression of LEF1 in colon cancer. Mol Cell Biol 26(14):5284–5299. https://doi.org/10.1128/MCB.00105-06
Santiago L, Daniels G, Wang D, Deng FM, Lee P (2017) Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res 7(6):1389–1406
Vallee A, Lecarpentier Y, Vallee JN (2019) Targeting the canonical WNT/beta-catenin pathway in cancer treatment using non-steroidal anti-inflammatory drugs. Cells. https://doi.org/10.3390/cells8070726
Guo X, Wang XF (2009) Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res 19(1):71–88. https://doi.org/10.1038/cr.2008.302
Amini Nik S, Ebrahim RP, Van Dam K, Cassiman JJ, Tejpar S (2007) TGF-beta modulates beta-Catenin stability and signaling in mesenchymal proliferations. Exp Cell Res 313(13):2887–2895. https://doi.org/10.1016/j.yexcr.2007.05.024
Liu W, Rui H, Wang J, Lin S, He Y, Chen M, Li Q, Ye Z, Zhang S, Chan SC, Chen YG, Han J, Lin SC (2006) Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J 25(8):1646–1658. https://doi.org/10.1038/sj.emboj.7601057
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96(3):319–328. https://doi.org/10.1016/s0092-8674(00)80545-0
Galliher AJ, Schiemann WP (2006) Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res 8(4):R42. https://doi.org/10.1186/bcr1524
Mamuya FA, Duncan MK (2012) aV integrins and TGF-beta-induced EMT: a circle of regulation. J Cell Mol Med 16(3):445–455. https://doi.org/10.1111/j.1582-4934.2011.01419.x
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10(1):9–22. https://doi.org/10.1038/nrc2748
Belo VA, Guimaraes DA, Castro MM (2015) Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J Vasc Res 52(4):221–231. https://doi.org/10.1159/000441621
Sato A, Nagase H, Obinata D, Fujiwara K, Fukuda N, Soma M, Yamaguchi K, Kawata N, Takahashi S (2013) Inhibition of MMP-9 using a pyrrole-imidazole polyamide reduces cell invasion in renal cell carcinoma. Int J Oncol 43(5):1441–1446. https://doi.org/10.3892/ijo.2013.2073
Chen SJ, Yao XD, Peng BO, Xu YF, Wang GC, Huang J, Liu M, Zheng JH (2016) Epigallocatechin-3-gallate inhibits migration and invasion of human renal carcinoma cells by downregulating matrix metalloproteinase-2 and matrix metalloproteinase-9. Exp Ther Med 11(4):1243–1248. https://doi.org/10.3892/etm.2016.3050
Jang HS, Lal S, Greenwood JA (2010) Calpain 2 is required for glioblastoma cell invasion: regulation of matrix metalloproteinase 2. Neurochem Res 35(11):1796–1804. https://doi.org/10.1007/s11064-010-0246-8
Li X, Yang Y, Hu Y, Dang D, Regezi J, Schmidt BL, Atakilit A, Chen B, Ellis D, Ramos DM (2003) Alphavbeta6-Fyn signaling promotes oral cancer progression. J Biol Chem 278(43):41646–41653. https://doi.org/10.1074/jbc.M306274200
Niu J, Gu X, Turton J, Meldrum C, Howard EW, Agrez M (1998) Integrin-mediated signalling of gelatinase B secretion in colon cancer cells. Biochem Biophys Res Commun 249(1):287–291. https://doi.org/10.1006/bbrc.1998.9128
Li Y, Ren Z, Wang Y, Dang YZ, Meng BX, Wang GD, Zhang J, Wu J, Wen N (2018) ADAM17 promotes cell migration and invasion through the integrin beta1 pathway in hepatocellular carcinoma. Exp Cell Res 370(2):373–382. https://doi.org/10.1016/j.yexcr.2018.06.039
Yang L, Song X, Zhu J, Li M, Ji Y, Wu F, Chen Y, Cui X, Hu J, Wang L, Cao Y, Wei Y, Zhang W, Li F (2017) Tumor suppressor microRNA-34a inhibits cell migration and invasion by targeting MMP-2/MMP-9/FNDC3B in esophageal squamous cell carcinoma. Int J Oncol 51(1):378–388. https://doi.org/10.3892/ijo.2017.4015
Peng X, Zhang Q, Zeng Y, Li J, Wang L, Ai P (2015) Evodiamine inhibits the migration and invasion of nasopharyngeal carcinoma cells in vitro via repressing MMP-2 expression. Cancer Chemother Pharmacol 76(6):1173–1184. https://doi.org/10.1007/s00280-015-2902-9
Chen YS, Meng F, Li HL, Liu QH, Hou PF, Bai J, Zheng JN (2016) Dicer suppresses MMP-2-mediated invasion and VEGFA-induced angiogenesis and serves as a promising prognostic biomarker in human clear cell renal cell carcinoma. Oncotarget 7(51):84299–84313. https://doi.org/10.18632/oncotarget.12520
Zhang YE (2017) Non-Smad signaling pathways of the TGF-beta family. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a022129
Funding
This study was supported by National Science Foundation of China (No. 81572502).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Shang, D. Transforming growth factor-β1 enhances proliferative and metastatic potential by up-regulating lymphoid enhancer-binding factor 1/integrin αMβ2 in human renal cell carcinoma. Mol Cell Biochem 465, 165–174 (2020). https://doi.org/10.1007/s11010-019-03676-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03676-8