Abstract
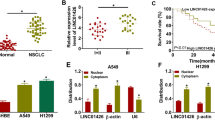
Non-small cell lung cancer (NSCLC) is the main subtype of lung cancer. The overall survival of NSCLC patients is relatively low even after various treatments. Accumulating evidence demonstrated that long non-coding RNA (LncRNA) plays crucial roles in different biological process. However, the role of a novel LncRNA, LINC00243, in NSCLC remains unclear. We aimed to explore the biological role of LINC00243 in NSCLC. The mRNA and protein levels were determined by real-time PCR and western blot, respectively. Cell viability in vitro was detected by Cell Counting Kit-8 (CCK-8) assay and colony-formation assay. Reporter assay was used to detect the interactions between molecules, and the interaction was assessed by RNA pull-down assay. LINC00243 expression increased in human NSCLC tissues and associated with poor prognosis of NSCLC patients. LINC00243 knockdown inhibited proliferation and glycolysis of NSCLC cells. Mechanically, LINC00243 acted as a molecular sponge for miR-507, and miR-507 reversed the effect of LINC00243 on promoting proliferation and glycolysis of NSCLC cells. Moreover, LINC00243 modulated expression of endogenous miR-507-targeted PDK4. LINC00243 promotes proliferation and glycolysis in NSCLC cells by positively regulating PDK4 through sponging miR-507. LINC00243 could be the potential target for NSCLC treatment clinically.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Zappa C, Mousa SA (2016) Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 5:288–300. https://doi.org/10.21037/tlcr.2016.06.07
Herbst RS, Heymach JV, Lippman SM (2008) Lung cancer. N Engl J Med 359:1367–1380. https://doi.org/10.1056/NEJMra0802714
Zakaria N, Satar NA, Abu Halim NH, Ngalim SH, Yusoff NM, Lin J, Yahaya BH (2017) Targeting lung cancer stem cells: research and clinical impacts. Front Oncol 7:80. https://doi.org/10.3389/fonc.2017.00080
Lo Russo G, Proto C, Garassino MC (2016) Afatinib in the treatment of squamous non-small cell lung cancer: a new frontier or an old mistake? Transl Lung Cancer Res 5:110–114. https://doi.org/10.3978/j.issn.2218-6751.2015.12.02
Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R (2016) FDA approval summary: nivolumab for the treatment of metastatic non-small cell lung cancer with progression on or after platinum-based chemotherapy. Oncologist 21:634–642. https://doi.org/10.1634/theoncologist.2015-0507
Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, Rizvi NA (2016) Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol 17:299–308. https://doi.org/10.1016/s1470-2045(15)00544-6
Fenchel K, Sellmann L, Dempke WC (2016) Overall survival in non-small cell lung cancer-what is clinically meaningful? Transl Lung Cancer Res 5:115–119. https://doi.org/10.3978/j.issn.2218-6751.2016.01.06
Choi SY, Collins CC, Gout PW, Wang Y (2013) Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol 230:350–355. https://doi.org/10.1002/path.4218
Lopez-Saez JF, de la Torre C, Pincheira J, Gimenez-Martin G (1998) Cell proliferation and cancer. Histol Histopathol 13:1197–1214. https://doi.org/10.14670/hh-13.1197
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L (2013) The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology 2:e22058. https://doi.org/10.4161/onci.22058
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64:3892–3899. https://doi.org/10.1158/0008-5472.Can-03-2904
Quinn JJ, Chang HY (2016) Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17:47–62. https://doi.org/10.1038/nrg.2015.10
Diermeier SD, Chang KC, Freier SM, Song J, El Demerdash O, Krasnitz A, Rigo F, Bennett CF, Spector DL (2016) Mammary tumor-associated RNAs impact tumor cell proliferation, invasion, and migration. Cell Rep 17:261–274. https://doi.org/10.1016/j.celrep.2016.08.081
Guan YJ, Ma JY, Song W (2019) Identification of circRNA-miRNA-mRNA regulatory network in gastric cancer by analysis of microarray data. Cancer Cell Int 19:183. https://doi.org/10.1186/s12935-019-0905-z
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899. https://doi.org/10.1038/nrc1478
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Kung JT, Colognori D, Lee JT (2013) Long noncoding RNAs: past, present, and future. Genetics 193:651–669. https://doi.org/10.1534/genetics.112.146704
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464. https://doi.org/10.1146/annurev-cellbio-092910-154237
Hu Y, Lu W, Chen G, Wang P, Chen Z, Zhou Y, Ogasawara M, Trachootham D, Feng L, Pelicano H, Chiao PJ, Keating MJ, Garcia-Manero G, Huang P (2012) K-ras(G12 V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res 22:399–412. https://doi.org/10.1038/cr.2011.145
Di Leva G, Garofalo M, Croce CM (2014) MicroRNAs in cancer. Annu Rev Pathol 9:287–314. https://doi.org/10.1146/annurev-pathol-012513-104715
Wang J, Qian Y, Gao M (2019) Overexpression of PDK4 is associated with cell proliferation, drug resistance and poor prognosis in ovarian cancer. Cancer Manag Res 11:251–262. https://doi.org/10.2147/cmar.S185015
Deng YH, Deng ZH, Hao H, Wu XL, Gao H, Tang SH, Tang H (2018) MicroRNA-23a promotes colorectal cancer cell survival by targeting PDK4. Exp Cell Res 373:171–179. https://doi.org/10.1016/j.yexcr.2018.10.010
Li G, Li M, Hu J, Lei R, Xiong H, Ji H, Yin H, Wei Q, Hu G (2017) The microRNA-182-PDK4 axis regulates lung tumorigenesis by modulating pyruvate dehydrogenase and lipogenesis. Oncogene 36:989–998. https://doi.org/10.1038/onc.2016.265
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study was approved by the ethics committee of the 900 Hospital of the Joint Logistics Team.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, X., Yang, S. Long non-coding RNA LINC00243 promotes proliferation and glycolysis in non-small cell lung cancer cells by positively regulating PDK4 through sponging miR-507. Mol Cell Biochem 463, 127–136 (2020). https://doi.org/10.1007/s11010-019-03635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03635-3