Abstract
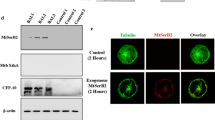
The genome sequence of Mycobacterium tuberculosis revealed the presence of several hydrolases involved in lipid metabolism including the members of Lip gene family. Rv0646c (LipG) is one of them. It is annotated as putative esterase/lipase because of the presence of consensus sequence ‘GXSXG.’ The gene was cloned, expressed, and purified in E. coli. It showed 22 U/mg specific activity with pNP-butyrate as a preferred substrate. However, it actively worked on substrates with short chain. The enzyme was optimally active at 50 °C/pH 8.0 and also stable up to 50 °C and in a lower pH range (pH 6–8). The Km, Vmax, and catalytic efficiency of the enzyme were calculated to be 500 µM, 58.82 µmoles/min/ml, and 3.92 µM/min, respectively. Homology modeling of Rv0646c revealed the presence of a canonical putative catalytic triad (Ser123, His279, and Asp251). The esterase activity was abolished in the presence of serine hydrolase inhibitors, THL and PMSF. Various antigenic epitopes were predicted in Rv0646c. The protein mounted significantly high antibody response against the sera of extrapulmonary and MDR-TB patients. Rv0646c up-regulated the production of various pro-inflammatory cytokines (TNF-α and IFN-γ), chemokine (IL-8), and nitric oxide in THP-1-derived macrophages. The secretion of IL-6 from macrophages was also found to be elevated in response to Rv0646c. The treatment resulted in the increased level of reactive oxygen species. Conclusively, Rv0646c could be classified as esterase having vast immunogenic property by eliciting strong humoral response as well as cell-mediated immunity.








Similar content being viewed by others
References
Williams DL, Torrero M, Wheeler PR, Truman RW, Yoder M, Morrison N et al (2004) Biological implications of Mycobacterium leprae gene expression during infection. J Mol Microbiol Biotechnol 8:58–72
Rodríguez JG, Hernández AC, Helguera-Repetto C, Ayala DA, Guadarrama-Medina R, Anzóla JM et al (2014) Global adaptation to a lipid environment triggers the dormancy- related phenotype of Mycobacterium tuberculosis. MBio 5:1–13
Pawelczyk J, Viljoen A, Kremer L, Dziadek J (2017) The influence of AccD5 on AccD6 carboxyltransferase essentiality in pathogenic and non-pathogenic Mycobacterium. Sci. Rep 7:1–13. https://doi.org/10.1038/srep42692
Marrakchi H, Lanéelle MA, Daffé M (2014) Mycolic acids: structures, biosynthesis, and beyond. Chem Biol 21:67–85
Singh G, Kumar A, Maan P, Kaur J (2017) Cell wall associated factors of Mycobacterium tuberculosis as major virulence determinants: current perspectives in drugs discovery and design. Curr Drug Targets 18(16):1904–1918. https://doi.org/10.2174/1389450118666170711150034
Neyrolles O, Guilhot C (2011) Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis 91:187–195. https://doi.org/10.1016/j.tube.2011.01.002
Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. https://doi.org/10.1038/31159
Farrell M, Foster J, Holland T (1993) Molecular analysis and expression of the lipase of Staphylococcus epidermidis. J Gen Microbiol 139:267–277
Gribbon EM, Cunliffe WJ, Holland KT (1993) Interaction of Propionibacterium acnes with skin lipids in vitro. J Gen Microbiol 139:1745–1751
Schofield DA, Westwater C, Warner T, Balish E (2005) Differential Candida albicans lipase gene expression during alimentary tract colonization and infection. FEMS Microbiol Lett 244:359–365
Zvi A, Ariel N, Fulkerson J, Sadoff JC, Shafferman A (2008) Whole genome identification of Mycobacterium tuberculosis vaccine candidates by comprehensive data mining and bioinformatic analyses. BMC Med Genom 1:18
Canaan S, Maurin D, Chahinian H, Pouilly B, Durousseau C, Frassinetti F et al (2004) Expression and characterization of the protein Rv1399c from Mycobacterium tuberculosis: a novel carboxyl esterase structurally related to the HSL family. Eur J Biochem 271:3953–3961
Kaur G, Saini V, Kumari B, Kaur J, Kaur J (2017) Characterization of an extracellular protein, Rv1076 from M. tuberculosis with a potential role in humoral response. Int J Biol Macromol 101:621–629. https://doi.org/10.1016/j.ijbiomac.2017.03.096
Jadeja D, Dogra N, Arya S, Singh G, Singh G, Kaur J (2016) Characterization of LipN (Rv2970c) of Mycobacterium tuberculosis H37Rv and its probable role in xenobiotic degradation. J Cell Biochem 117:390–401
Saxena AK, Roy KK, Singh S, Vishnoi SP, Kumar A, Kashyap VK et al (2013) Identification and characterisation of small-molecule inhibitors of Rv3097c-encoded lipase (LipY) of Mycobacterium tuberculosis that selectively inhibit growth of bacilli in hypoxia. Int J Antimicrob Agents 42:27–35. https://doi.org/10.1016/j.ijantimicag.2013.03.007
Shen G, Singh K, Chandra D, Carole SA, Maurin D, Canaan S et al (2012) LipC (rv0220) is an immunogenic cell surface esterase of Mycobacterium tuberculosis. Infect Immun 80:243–253
Singh G, Kumar A, Arya S, Gupta UD, Singh K, Kaur J (2016) Characterization of a novel esterase Rv1497 of Mycobacterium tuberculosis H37Rv demonstrating β-lactamase activity. Enzyme Microb Technol 82:180–190
Singh G, Arya S, Manisha, Kaur J (2014) Rv2485c, a putative lipase of M. tuberculosis: expression, purification biochemical characterization. Int J Trop Dis Health 4:1–17
Singh G, Arya S, Narang D, Jadeja D, Singh G, Gupta UD et al (2014) Characterization of an acid inducible lipase Rv3203 from Mycobacterium tuberculosis H37Rv. Mol Biol Rep 41:285–296
Srinivas M, Rajakumari S, Narayana Y, Joshi B, Katoch VM, Rajasekharan R et al (2008) Functional characterization of the phospholipase C activity of Rv3487c and its localization on the cell wall of Mycobacterium tuberculosis. J Biosci 33:221–230
Singh G, Arya S, Kumar A et al (2014) Molecular characterization of oxidative stress-inducible LipD of Mycobacterium tuberculosis H37Rv. Curr Microbiol 68(3):387–396
Brust B, Lecoufle M, Tuaillon E, Dedieu L, Canaan S, Valverde V et al (2011) Mycobacterium tuberculosis lipolytic enzymes as potential biomarkers for the diagnosis of active tuberculosis. PLoS ONE 6:1–11
Kumar A, Singh SM, Singh R, Kaur J (2017) Rv0774c, an iron stress inducible, extracellular esterase is involved in immune-suppression associated with altered cytokine and TLR2 expression. Int J Med Microbiol 307:126–138. https://doi.org/10.1016/j.ijmm.2017.01.003
Kumar A, Saini V, Kumar A, Kaur J, Kaur J (2017) Modulation of trehalose dimycolate and immune system by Rv0774c protein enhanced the intracellular survival of Mycobacterium smegmatis in human macrophages cell line. Front Cell Infect Microbiol. 7:289. https://doi.org/10.3389/fcimb.2017.00289/abstract
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Kaur J, Kumar A, Kaur J (2018) Strategies for optimization of heterologous protein expression in E. coli: Roadblocks and reinforcements. Int J Biol Macromol 106:803–822. https://doi.org/10.1016/j.ijbiomac.2017.08.080
Sambrook J, Fritsch EF, Maniatis T(1989) Molecular cloning: a laboratory manual. Cold Spring Harbour, New York, pp. 1–2
Dosanjh NS, Kaur J (2002) Biochemical analysis of a native and proteolytic fragment of a high-molecular-weight thermostable lipase from a mesophilic Bacillus sp. Protein Expr Purif 24:71–75
Sarkar M, Varshney R, Chopra M, Sekhri T, Adhikari JS, Dwarakanath BS (2005) Flow-cytometric analysis of reactive oxygen species in peripheral blood mononuclear cells of patients with thyroid dysfunction. Cytom B 23:20–23
Yamamoto K, Akbar F, Masumoto T, Onji M (1998) Increased nitric oxide (NO) production by antigen-presenting dendritic cells is responsible for low allogeneic mixed leucocyte reaction (MLR) in primary biliary cirrhosis (PBC). Clin Exp Immunol 114:94–101
Kuhn ML, Alexander E, Minasov G, Page HJ, Warwrzak Z, Shuvalova L et al (2016) Structure of the essential Mtb FadD32 enzyme: a promising drug target for treating tuberculosis. ACS Infect Dis 2:579–591
Singh G, Kumar A, Arya S, Gupta UD, Singh K, Kaur J (2016) Characterization of a novel esterase Rv1497 of Mycobacterium tuberculosis H37Rv demonstrating β-lactamase activity. Enzyme Microb Technol 82:180–90. https://doi.org/10.1016/j.enzmictec.2015.10.007
Yeruva VC, Savanagouder M, Khandelwal R, Kulkarni A, Sharma Y, Raghunand TR (2016) The Mycobacterium tuberculosis desaturase DesA1 (Rv0824c) is a Ca2 + binding protein. Biochem Biophys Res Commun 480:29–35
Zhang L, Skolnick J (1998) What should the Z-score of native protein structures be? Protein Sci 7:1201–1207
Gu S, Chen J, Dobos KM, Bradbury EM, Belisle JT, Chen X (2003) Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol Cell Proteom 2:1284–1296
Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C et al (2010) Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med 2:258–274
Kaur G, Kaur J (2017) Multifaceted role of lipids in Mycobacterium leprae. Future Microbiol 12:315–335. http://www.ncbi.nlm.nih.gov/pubmed/28287297
Kumar A, Sharma A, Kaur G, Makkar P, Kaur J (2017) Functional characterization of hypothetical proteins of Mycobacterium tuberculosis with possible esterase/lipase signature: a cumulative in silico and in vitro approach. J Biomol Struct Dyn 35:1226–1243. http://www.ncbi.nlm.nih.gov/pubmed/27050490
Lin Y, Li Q, Xie L, Xie J (2017) Mycobacterium tuberculosis rv1400c encodes functional lipase/esterase. Protein Expr Purif 129:143–149. https://doi.org/10.1016/j.pep.2016.04.013
Brennan PJ (1984) Mycobacterium leprae—the outer lipoidal surface. J Biosci 6:685–689
Brennan PJ (2003) Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83:91–97
Briken V, Porcelli SA, Besra GS, Kremer L (2004) Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol 53:391–403
Brosch R, Gordon SV, Pym A, Eiglmeier K, Garnier T, Cole ST (2000) Comparative genomics of the mycobacteria. Int J Med Microbiol 290:143–152. http://linkinghub.elsevier.com/retrieve/pii/S1438422100800831
Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C (1999) Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol 34:257–267
Lamichhane G (2011) Mycobacterium tuberculosis response to stress from reactive oxygen and nitrogen species. Front Microbiol 2:1–2
Moon M, Kim S, Moon H, Kim D (2017) Mycobacterium Tuberculosis in Spinal Tuberculosis. Asian Spine J 11:138–149
Yang C-S, Lee H-M, Lee J-Y, Kim J-A, Lee SJ, Shin D-M et al (2007) Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J Neuroinflammation 4:27. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2235845&tool=pmcentrez&rendertype=abstract
Hu S, He W, Du X, Yang J, Wen Q, Zhong X-P et al (2017) IL-17 production of neutrophils enhances antibacteria ability but promotes arthritis development during Mycobacterium tuberculosis infection. EBioMedicine 23:88–89. https://doi.org/10.1016/j.ebiom.2017.08.001
Cavalcanti YVN, Brelaz MCA, Neves JKDAL, Ferraz JC, Pereira VRA (2012) Role of TNF-alpha, IFN-gamma, and IL-10 in the development of pulmonary tuberculosis. Pulm Med 2012:745483. https://doi.org/10.1155/2012/745483
Sharma S, Sharma M, Roy S, Kumar P, Bose M (2004) Mycobacterium tuberculosis induces high production of nitric oxide in coordination with production of tumour necrosis factor-α in patients with fresh active tuberculosis but not in MDR tuberculosis. Immunol. Cell Biol 82:377–382
Ameixa C, Friedland JS (2002) Interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes is regulated by protein tyrosine kinases but not by ERK1 / 2 or p38 mitogen-activated protein kinases interleukin-8 secretion from Mycobacterium tuberculosis-infected monocytes. Infect Immun 70:4743–4746
Singh PP, Goyal A (2013) Interleukin-6: a potent biomarker of mycobacterial infection. Springerplus 2:686
Choudhary RK, Mukhopadhyay S, Chakhaiyar P, Sharma N, Murthy KJR, Katoch VM et al (2003) PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect Immun 71:6338–6343
Mishra KC, De Chastellier C, Narayana Y, Bifani P, Brown AK, Besra GS et al (2008) Functional role of the PE domain and immunogenicity of the Mycobacterium tuberculosis triacylglycerol hydrolase LipY. Infect Immun 76:127–140
Singh KK, Dong Y, Patibandla SA, McMurray DN, Arora VK, Laal S (2005) Immunogenicity of the Mycobacterium tuberculosis PPE55 (Rv3347c) protein during incipient and clinical tuberculosis. Infect Immun 73:5004–5014
Almeida PE, Silva AR, Maya-Monteiro CM, Torocsik D, D’Avila H, Dezso B et al. Mycobacterium bovis Bacillus Calmette-Guerin Infection Induces TLR2-dependent peroxisome proliferator-activated receptor expression and activation: functions in inflammation, lipid metabolism, and pathogenesis. J Immunol 183:1337–1345. http://www.ncbi.nlm.nih.gov/pubmed/19561094
Pieters J (2008) Mycobacterium tuberculosis and the macrophage: maintaining a balance. Cell Host Microbe 3:399–407. http://www.ncbi.nlm.nih.gov/pubmed/18541216
Yadav M, Roach SK, Schorey JS (2004) Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol 172:5588–5597. http://www.ncbi.nlm.nih.gov/pubmed/15100302
Li W, Zhao Q, Deng W, Chen T, Liu M, Xie J (2014) Mycobacterium tuberculosis Rv3402c enhances mycobacterial survival within macrophages and modulates the host pro-inflammatory cytokines production via NF-kappa B/ERK/p38 signaling. PLoS ONE 9:e94418. https://doi.org/10.1371/journal.pone.0094418
Kim K, Sohn H, Kim J-S, Choi H-G, Byun E-H, Lee K-I et al (2012) Mycobacterium tuberculosis Rv0652 stimulates production of tumour necrosis factor and monocytes chemoattractant protein-1 in macrophages through the Toll-like receptor 4 pathway. Immunology 136:231–240. http://www.ncbi.nlm.nih.gov/pubmed/22385341
Denis M, Wedlock DN, Buddle BM (2005) IFN-gamma enhances bovine macrophage responsiveness to Mycobacterium bovis: impact on bacterial replication, cytokine release and macrophage apoptosis. Immunol Cell Biol 83:643–650. http://www.ncbi.nlm.nih.gov/pubmed/16266317
Carvalho-Pinto CE, García MI, Mellado M, Rodríguez-Frade JM, Martín-Caballero J, Flores J et al (2002) Autocrine production of IFN-gamma by macrophages controls their recruitment to kidney and the development of glomerulonephritis in MRL/lpr mice. J Immunol 169:1058–1067. http://www.ncbi.nlm.nih.gov/pubmed/12097414
Gessani S, Belardelli F (1998) IFN-gamma expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev 9:117–123. http://www.ncbi.nlm.nih.gov/pubmed/9754706
Acknowledgements
ICMR fellowship to Ruchi Rastogi (File No. 80/839/2013/ECD-1) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest to this work.
Additional information
Ruchi Rastogi and Arbind Kumar have contributed equally for this publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rastogi, R., Kumar, A., Kaur, J. et al. Rv0646c, an esterase from M. tuberculosis, up-regulates the host immune response in THP-1 macrophages cells. Mol Cell Biochem 447, 189–202 (2018). https://doi.org/10.1007/s11010-018-3303-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-018-3303-2