Abstract
Venom and poison peptides are powerful biological weapons and have proven immense pharmacological potential because of their high binding affinity to a wide range of molecular targets. Nonetheless, many of these peptides cannot directly be used as medicines due to their toxicity but their derivatives are very valuable to explore and can be a great treasure trove for the development of novel drugs. This review presents a detailed overview of venom peptides present in reptiles, amphibians, arachnids, gastropods, clitellatas, fish, insects, and mammals. We address the most recent findings that underline their therapeutic potential against a wide variety of diseases from cancer to vascular, autoimmune, and inflammatory diseases.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Venoms vs. Poisons
First, it is important to conceptualize and distinguish the two terms: venom and poison. Both can lead to the victim death but are two different biological weapons. The major difference between venom and poison depends on how the toxins are secreted and transferred to the prey or to the victim. However, according to Jared and colleagues (Jared et al. 2021), in practice there is no consensus among theorists and naturalistic biologists regarding the use of both terms.
The authors support a more complete definition of both terms, in which, in addition to conventional and functional interpretation, include: (i) The type of chemical defensive behavior of the organism, whether passive or active; (ii) Venomous animals inject their toxins directly into the tissues and/or organs of their victims. Poisonous animals, on the other hand, usually release venom through their mucous membranes; (iii) The glands of active or passive organisms seem to show anatomical and morphological differences. In active defense, the glands communicate with teeth or fangs and are surrounded by muscles that assist the rapid injection of the venom. In passive defense organisms (such as in amphibians), the action promoted by aggression, for example through bites, provokes the ejection or release of the poison that first reaches the mucous membranes or the skin, indirectly accessing the blood circulation. In this sense, for this editorial we will consider both terms in an integrated manner, as previously proposed.
Venomous and Poisonous Animals
Venomous and poisonous animals are those capable of producing toxic secretions for both protection and predation. These animals are widely distributed throughout the world, but envenomation accidents occur mainly in tropical and subtropical regions where the abundance of species is greater (Oliveira et al. 2022).
Snakes and scorpions are the envenoming protagonists responsible for the most serious accidents. Snakes alone cause an estimated 2.7 million envenomings every year and up to 138 thousand deaths (Chippaux 2008). As a result, the World Health Organization (WHO) has included snake bite envenomation in the list of neglected tropical diseases, noting that a new approach to preventing and treating snake bite envenomation is urgently needed (Chippaux et al. 2019; Basnyat and Shilpakar 2022).
Venoms are produced by thousands of species in the animal kingdom, including annelids (barbed fireworm), cnidarians (sea anemones and jellyfish), echinoderms (sea urchins and starfish), mollusks (snails and octopuses), arthropods (spiders, ants, bees, wasps, scorpions, mosquitoes and ticks) and vertebrates (fish, frogs, snakes, lizards, birds and mammals) (Pennington et al. 2018; Luddecke et al. 2022; Mackessy 2022; Menezes and Thakur 2022).
Each animal venom is unique. Venoms consist of a species-specific heterogeneous complex mix, often a mixture of peptides, enzymes, low molecular weight organic molecules, and inorganic salts (Simoes-Silva et al. 2018). All molecules present in the venom have physicochemical and biological properties that result from an evolutionary process, providing such animals an efficient mechanism of subjugation and feeding, which also acts as a defense weapon (Guimaraes et al. 2014; Estevao-Costa et al. 2018; Fischer and Riedl 2022).
Venoms: Villains or Heroes?
The mere sight of venom animals strikes terror in the hearts of millions of people, but venom has been used in traditional medicine for centuries. It is true that accidents with venomous animals, even mild ones, can lead to death. However, because of their pharmacologic potential, venom peptides (and other molecules) are also of great use to humanity and save many more lives than they take (Almeida et al. 2016; Correa et al. 2016; Charitos et al. 2022; Oliveira et al. 2022; Diniz-Sousa et al. 2023). Peptide toxins have high affinity to various molecular targets such as enzymes, transmembrane receptors, and cell membranes. They destabilize the victims or prey physiological systems which are essential for their survival (Calvete 2018). As a result, venom peptides exhibit hemotoxic, cytotoxic and/or neurotoxic effects, among others, achieved by specific interactions between the toxin and its target (Warrell 2017, 2019; Akef 2018; Simoes-Silva et al. 2018; Almeida et al. 2019). Due to the ability of toxins to interact with key physiological targets, which are fundamental to homeostasis, many peptides from animal venoms have the potential to produce prototype therapeutic molecules that can help organisms restore their normal functions.
Thus, it is easily conceivable that such molecules can be used as powerful therapeutics in appropriate doses and in the right context (Herzig et al. 2020). In fact, many isolated peptides from venom toxins showed therapeutic potential exhibiting antitumor, antimicrobial, anticoagulant, or analgesic activities (Fig. 1) (Correa et al. 2016; Diniz-Sousa et al. 2018; Simoes-Silva et al. 2018; da Silva Caldeira et al. 2021).
Examples of peptides derived from animal venoms that present therapeutic potential. In the figure are indicated 10 animals: snakes, spiders, scorpions, bees, amphibians, leeches, lizards, fish, shrews, and snails, and their molecular and cellular targets (A to F) A neuronal cells; B muscular cells; C cancer cells; D blood cells; E bacterial cell; and F pancreatic cell. Figure created with BioRender.com (2020) (https://app.biorender.com/biorender-templates)
With the advent of protein structural techniques and the refinement of isolation and characterization methods, it became possible to clarify the structure and pharmacological activities of these bioactive molecules and to better understand venom toxins. Such studies have helped elucidate toxin interactions with specific molecular targets and led to effective drugs for various pathologies (Almeida et al. 2017, 2018, 2019; Utkin 2017; Mendes et al. 2019).
It is important to mention that while snakes and lizards have large venom glands that easily produce enough venom quantities for biochemical, pharmacological, structural, and functional studies, other venomous animals such as spiders, scorpions and bees produce much smaller amounts, which has long been a challenge to study. Current sequencing technologies, peptide engineering and the latest computational tools have greatly overcome these obstacles. In fact, after 1980, many drugs were developed from animal venom toxins and approved by the Food and Drug Administration ( FDA) and the European Medicines Agency ( EMA) (Ma et al. 2017; Bordon et al. 2020; Coulter-Parkhill et al. 2021) (Table 1). This review summarizes the most relevant animal venom peptides that have led to powerful drugs or are currently being evaluated in clinical or preclinical trials.
Reptiles and Clitellatas that inspired commercial drugs. A Bothrops moojeni (Batroxobin). B Naja naja atra (Cobratide). C Hirudo medicinalis (Desirudin, Bivalirudin and Leech terapia). D Heloderma suspectum (Exenatide and Lixisenatide). Photos available at CalPhotos (https://calphotos.berkeley.edu). The authorship and ownership of the photographs in indicated under each photo
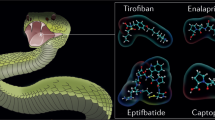
The three drugs derivate from snake venom peptides in complex with their specific targets: Tirofiban® (PDB: 7TD8) (Zhu et al. 2022b) and Eptifibatide® bound to the αIIBβ3 integrin (PDB: 7THO) (Zhu et al. 2022a) and Captopril® blocking the angiotensin-converting enzyme active site (PDB: 1UZF) (Natesh et al. 2004). Figure was created with BioRender.com (2020) (https://app.biorender.com/biorender-templates)
Disintegrins derived drugs that have been approved by the FDA and EMA. The figure shows on the top a diagram of the peptides primary structure, where is highlighted the integrin recognition motif and the sulfur bridges, characteristic of this peptide family. It is also shown the secondary structure of these Viperidae toxins, where the residues pinpointed in the diagram are represented as spheres and sticks. On the bottom is shown the chemical structure of the medicines and the moieties that replicate the biopolymer. A Echistatin from Echis Carinatus (PDB ID. 2ECH) binds specifically to the αIIBβ3 integrin by the RGD motif and inspired the synthesis of Tirofiban. The (S)-NHSO2C4H9 group increases the binding affinity of tirofiban for its target. B Barbourim from Sistrurus miliarius barbourin (Alpha fold model AF-P22827-F1) also binds the αIIBβ3 integrin and inspired the synthesis of eptifibatide. The last accomplishes maximum selectivity by melding two motifs into the unnatural homoRDG motif
The snake Viperidae family. A The Brazilian viper B. jararaca led to the discovery of Captopril. B The Indian saw-scaled viper: E. carinatus and C the pygmy rattlesnake—Sistrurus miliarius—inspired the anti-platet drugs Tirofiban and Eptifibatide, respectively. The authorship and ownership of each is indicated on each photograph. Photographs A and C are available at CalPhotos (https://calphotos.berkeley.edu). Photograph B is curtesy of P. Gowri Shankar from Kalinga Foundation, India. The authorship and ownership of the photographs in indicated under each photo
Toxin Peptides from Animal Kingdom: Therapeutics and Diagnostic Potential
Snakes
Animal venom-derived peptides are a potential source of inspiration to produce new drugs and therapies. Toxins isolated from snake venom are a great example of molecules that led to important drugs in clinical practice. In fact, there are eight small synthetic drugs inspired by snake venom toxins approved by the FDA and EMA, which mainly aim to treat cardiovascular disease, a global public health problem that causes more than 18 million deaths a year. Captopril (Fig. 3; Table 1) was the first drug to be developed from snake venom. It is an antihypertensive molecule used to control blood pressure by inhibiting the angiotensin-converting enzyme (ACE) (Natesh et al. 2004; Camargo et al. 2012; Harvey 2014). Captopril is a drug derived from bradykinin-enhancing peptides isolated from the venom of the Brazilian jararaca (Bothrops jararaca), see Fig. 5A. The drug was approved by the FDA in 1981 and in Europe in 1984. Since then, it has become a blockbuster drug with an annual turnover of more than $1 billion. After all these years, captopril, and its derivatives, such as enalapril, ramipril, and lisinopril, remain among the four most prescribed drugs worldwide for hypertension (Table 1) (Mohamed Abd El-Aziz et al. 2019; Bordon et al. 2020).
Other relevant snake venom component with therapeutic interest are the disintegrins. These small cysteine-rich peptides are often found in viper venom and can develop severe toxic effects due to their high affinity for the α and ß subunits of the integrins, a cellular membrane receptor directly associated with the integrity of mammalian tissues. Disintegrins act as powerful integrin antagonists because they recognize integrins based on a tripeptide motif located on a specific binding loop of disintegrins. Well-known examples of the tripeptide recognition motifs are: the RGD motif, present on Echistatin and Bitistatin, the KGD motif of Barbuorin, and KTS motif of Obtustatin. Other similar tripeptide motifs present on disintegrins are: MLD, VGD, MGD, WGD, MVD and RTS. (Almeida et al. 2016; Mohamed Abd El-Aziz et al. 2019; Boldrini-Franca et al. 2020; Trim et al. 2021; Chan et al. 2022; Siigur and Siigur 2022). Yet, the RGD motif deserves particular attention due to its ability to bind 8 out of the 24 known integrins—αIIbβ3, α5β1, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8—which comprises not only the integrins that bind fibrinogen, glycoproteins, fibronectin and thrombospondin, but also the 4 collagen receptors, 4 laminin receptors, and 8 leukocyte receptors (Notni 2022). In contrast, the KGD motif recognizes specifically the αIIbβ3 on the platelet surface (Bachmann et al. 2019), and KTS motif recognizes the α1β1 integrin receptor (Khamessi et al. 2022).
Onwards, we will discuss two disintegrins used to develop drugs for the treatment of acute coronary syndrome, namely tirofiban and eptifibatide (Frangieh et al. 2021; Vasconcelos et al. 2021). The first, tirofiban (Fig. 3; Table 1), is a non-peptide drug antagonist that recognizes and competes with the fibrinogen to bind to the αIIBß3 integrin on platelets, which hinders its aggregation. Tirofiban was inspired by the echistatin structure (Fig. 4A), a short monomeric disintegrin, that present a RGD motif and was isolated from the Echis carinatus venom (Saw-scaled viper, Fig. 5B) (Gan et al. 1988; Garsky et al. 1989; Lazarovici et al. 2019; Coulter-Parkhill et al. 2021).
The last, eptifibatide (Fig. 3; Table 1) was developed from barbourin, a KGD-motif disintegrin (Fig. 4B) isolated from the venom of the Barbour pygmy rattlesnake (Sistrurus miliarius barbourin), Fig. 5C. The final structure of eptifibatide is a cyclized heptapeptide that provides resistance to proteolysis and presents high affinity and specificity for the αIIBβ3 integrin (O'Shea and Tcheng 2002; Lazarovici et al. 2019; Mohamed Abd El-Aziz et al. 2019; Bordon et al. 2020; Trim et al. 2021). Both drugs were approved in 1998 by the FDA, and the EMA in 1999 and are good examples of the transformation of a venom peptide into a lifesaving drug (Table 1).
(Information based in Khan et al. 2018; Uzair et al. 2018; Cesar et al. 2019; Siigur et al. 2019; Oliveira et al. 2022; Teodoro et al. 2022; Yu et al. 2022).
Tirofiban and eptifibatide are examples of pharmaceutical success based on inhibition of adhesion processes by integrins. Adhesion processes are fundamental to the production of the tumor microenvironment and the development of cancer. The ability of disintegrins to inhibit adhesion processes has encouraged researchers to devote enormous efforts and financial resources to understanding how ophidic disintegrins can be used against cancer. In fact, disintegrins induce antitumoral effects such as antiproliferative, cytotoxic, proapoptotic, antiangiogenic, antiinvasive and antiadhesive effects. They also inhibit migration, metastasis and invasion in lung, breast, pancreatic, prostate and melanomas (Mullooly et al. 2016; Scharfenberg et al. 2020). Yet, a deep knowledge about disintegrins and their involvement in cancer-associated cell mechanisms is needed to explore their potential and produce novel anticancer drugs (Calderon et al. 2014; Huang et al. 2019; Zhang et al. 2022; Zhou et al. 2022). Some of those examples are shown in Table 2.
Boldrini-Franca et al. (2019) produced recombinant Collinein-1 (Fig. 1), a snake venom serine protease (SVSP) from the venom of Crotalus durissus collilineatus expressed in the Pichia pastoris system. Collinein-1 blocks the human ether-à-go-go ion channel (hEAG1), a voltage-gated potassium (Kv) subfamily, which is overexpressed in cancer cell lines and is implicated in the progression of tumor cells (Boldrini-Franca et al. 2019). Collinein-1 reduced the viability of the human breast cancer cell line MCF7, which exhibits high expression of hEAG1. However, the toxin has no effect on HepG2 (human liver cancer) and MCF10A (human breast cancer) cell lines that are low in expression of this ion channel. These findings suggest that the reduction in cell viability is related to hEAG1 by Collinein-1 (Bordon et al. 2020).
Crotamine (CTa) is a highly cationic and cysteine-rich peptide isolated from the venom of Crotalus durissis terrificus (Fig. 1), which shows membrane translocation capabilities, penetrates the cell and presents cytoplasmic, vesicular and nuclear distribution (Almeida et al. 2017; Pompeia et al. 2022; Si et al. 2022). CTa can be specifically uptaken by melanoma and lymphoma cells during their proliferation and permeates several lines in vitro (Si et al. 2022). Furthermore, CTa and its analogs have been tested in tumor cell lines and have demonstrated aptitude to be used as selective delivery tools for anticancer molecules (Mambelli-Lisboa et al. 2018). CTa is also a potential tool for in vivo cancer cell identification (Kerkis et al. 2014).
Crotalphine is a peptide isolated from C. durissis terrificus venom (Fig. 1) (Konno et al. 2008) and showed promising results against pain by acting on peripheral opioid receptors, and selectively targeting the transient receptor potential vanilloid 1 (TRPA1 ion channel) (Gutierrez et al. 2008; Zambelli et al. 2014; Bressan et al. 2016; Giardini et al. 2021; Lopes et al. 2022). The drug is a potent long-lasting opioid-mediated antinociception and has been evaluated for cancer pain (Brigatte et al. 2013; Xiong and Huang 2018; Giardini et al. 2021; Hayashi et al. 2022).
Another example is the chimeric natriuretic cenderitide (CD-NP), produced by the association of 15 amino acids of the C-terminal segment of a Dendroaspis angusticeps natriuretic peptide and 22 residues of the human C-type natriuretic peptide. CD-NP is indicated in congestive heart failure because it activates natriuretic peptide receptors, leading to hypotension. Cenderitide showed natriuretic, diuretic, antiproliferative, and antifibrotic proprieties. The drug is being evaluated in phase I/II trials for stable chronic heart failure and moderate renal impairment (Table 6) (Lee et al. 2016; Kawakami et al. 2018; Zhang et al. 2018a; Ichiki et al. 2019).
Spiders
Spiders are the biggest group of venomous animals. These animals are able to produce small peptide-rich venoms; each spider species can have thousands of different toxic peptides, which, given the sheer number of spider species (100–300,000 species), gives us a glimpse of the hidden richness of spider venom (Diego-Garcia et al. 2010; Peigneur et al. 2021). Since proteinaceous spider toxins are an invaluable resource for pharmacologists, neuroscientists and medical chemistry, scientists have created the ArachnoServer, a database that collects molecular targets, structural and biological information.
Most spider venom-derived act as analgesics because they are allosteric modulators of the Nav channels and are classified in twelve categories— NaSpTx 1–12—based on their primary sequence and cysteine positions (Goncalves-Machado et al. 2016; Luddecke et al. 2022; Trevisan and Oliveira 2022). A typical spider toxin has three disulfide bonds, which forms the Inhibitor Cysteine Knot (ICK) motif (C1–C4, C2–C5, and C3–C6), present in 9 out of the 10 NaSpTxs that specifically bind the Nav channels. (Esteves et al. 2020; Lima et al. 2021). However, spider toxins can also form 4 or even 5 disulfide bonds, as it is the case of the ω-agatoxin and the PnTxs, respectively. This structural aspect allows the peptides to interact with other channels involved in pain transmission, such as the acid-sensing ion channel (ASICs), calcium channels, opioids and purinergic receptors (Estrada et al. 2007; Kabanova et al. 2012; Pan et al. 2012, 2021; Utkin et al. 2019). This is indeed the case of the ω-agatoxins and the PnTxs which act as Cav channel modulators, blocking the presynaptic calcium channels, and thus, decreasing the calcium influx.
Table 3 sums up the main spider toxins that exhibit analgesic properties.
Below we describe the pharmacological relevance of other peptides present in spider venoms and their supporting studies for putative medical applications, many of which not only have analgesic properties, but also antimicrobial and anticancer activities (Perumal Samy et al. 2017):
-
(1)
ß-TRTX-Cd1a is a peptide isolated from Ceratogyrus darlingi (African rear-horned baboon tarantula). This peptide blocks Cav2.2 channels and Nav1.1–1.2 and Nav1.7–1.8, with a higher potency at Nav1.7. In vivo tests showed that β-TRTX-Cd1a can reverse OD1-induced pain in a mouse model of peripheral spontaneous pain (Sousa et al. 2017).
-
(2)
The venom of the Chinese tarantula (Chilobrachys jingzhao) expresses a family of peptides named Jingzhaotoxins (JzTx). The JzTx-34 peptide inhibits Nav1.7 channels, exhibiting antinociceptive properties with higher affinity compared to all other subtypes of Nav (Zeng et al. 2018).
-
(3)
Gomesin is a peptide isolated from the venom of Acanthoscurria gomesiana (São Paulo Black Tarantula) (Fig. 1). It has antimicrobial activity and reduces the viability and proliferation of human melanoma cells by inducing cell cycle arrest (Fernandez-Rojo et al. 2018; Ikonomopoulou et al. 2018).
-
(4)
LyeTxl-b is a potent antibiotic derived from the LyeTxl peptide found in the venom of Lycosa erythrognatha (Araña Lobo de Vientre Negro) (Fig. 1). It has proven antibacterial efficacy against Gram-positive and Gram-negative bacteria, in vitro and in vivo, and has delivered promising results against resistant bacteria strains. LyeTxl-b also reduced biofilm viability (Cardoso et al. 2022; de Avelar Junior et al. 2022).
-
(5)
Hi1a is a peptide isolated from Hadronyche infensa venom (Australian blue mountain funnel-web spider). Hi1a attenuates brain damage after a stroke and can be used in brain ischemic injuries (Chassagnon et al. 2017). Therefore, it is considered an important lead for the development of neuroprotective agents (Ren et al. 2018). Hi1a showed partial inhibition of ASIC1a but does not inhibit ASIC1b. The total venom of the Hadronyche infensa spider also contains biopesticides that paralyze insects associated with crop damage while being non-toxic against bees, birds and mammals (Kilham and Isbister 2020).
Scorpions
Scorpions are predatory arthropods and one of the oldest animals on Earth. They can be found all over the world, except Antarctica, but live mainly in deserts. Their venom is rich in neurotoxic peptides that interact with many ion channels, causing paralysis and other neurotoxic effects. Therefore, these peptides have been investigated to understand whether they can be used as prototype analgesic drugs. (Bagheri-Ziari et al. 2021; Tawfik et al. 2021; Trim et al. 2021; Soltan-Alinejad et al. 2022).
Chlorotoxin (CTx) is a positively charged peptide consisting of 36 amino acids and 4 disulfide bonds at physiological pH. CTx is an effective component of the venom of Leiurus quinquestriatus quinquestriatus (Fig. 6A) (the Deathstalker or yellow scorpion of the Middle East) because it can cause paralysis (Lippens et al. 1995; Jouirou et al. 2004; Morel et al. 2022). This peptide has the ability to bind to different targets including chloride channels, potassium channels membrane type-2 matrix metalloprotease (MMP-2) and annexin A2 (Fig. 1) (Kuzmenkov et al. 2018; Tajti et al. 2020; Zhu et al. 2020; Gandomkari et al. 2022). To date, CTx is the only scorpion venom toxin undergoing clinical trials due to its ability to bind to glioma cells, leading to the development of cancer diagnosis methods and treatment (Delinois et al. 2020; Zhao et al. 2020; Vannini et al. 2021; Dardevet et al. 2022).
Scorpions whose venoms have shown pharmacological activities. A the Deathstalker scorpion (Leiurus quinquestriatus) that led to discovery of the TM-601 and BLZ-100 peptides; B the Centruroides genus which led to the discovery of the Margatoxin, a peptide isolated from the venom of Centruroides margaritatus; and C The right the Androctonus genus; the Androctonus mauretanicus mauretanicus scorpion that produces the Kaliotoxin. Photos available at CalPhotos (https://calphotos.berkeley.edu). The authorship and ownership of the photographs in indicated under each photo
Examples of amphibians that produce antimicrobial peptides and insulin-releasing peptides. A The Rana bombina orientalis, which led to the discovery of BLPs and Bombilin-H. B The Rana grylio secretes the Temporins family toxins. C The Rana dybowskii secrete the Dybowskins. and D the Leptodactylus laticeps frog secretes the Plasticin-L1 toxin. Photos available at CalPhotos (https://calphotos.berkeley.edu). The authorship and ownership of the photographs in indicated under each photo
TM-601 is a synthetic derivative of CTx with proved antitumor characteristics. This drug candidate has undergone phase I and II clinical trials, for patients with recurrent malignant glioma and solid tumors (Jacoby et al. 2010; Kesavan et al. 2010; Ojeda et al. 2016; Dardevet et al. 2022). Another example is Tozuleristide (BLZ-100), also inspired on the CTx from the Leiurus quinquestriatus quinquestriatus scorpion (Fig. 6A). This CTx indocyanine green conjugate showed ability to bind to tumor cells. BLZ-100 can detect metastases (in mouse models) from prostate and intestinal cancers, malignant glioma and sarcoma medulloblastoma (Butte et al. 2014; Fidel et al. 2015; Parrish-Novak et al. 2017; Yamada et al. 2021). Furthermore, Hemilipins Hl-P11 and HL-P13 (Fig. 1), present in the venom of the scorpion Hemiscorpius lepturus, inhibit angiogenic activity and can be cytotoxic to breast and colorectal cancer cell lines (Jridi et al. 2015, 2017; Kazemi-Lomedasht et al. 2017; Parrish-Novak et al. 2017; Yamada et al. 2021; Rezaei et al. 2022). Many relevant toxin peptides present in the venom of various scorpion species can be used against channelopathies (ion channel associated pathologies), shown antimicrobial, antiangiogenic, anticancer, tumor binding or insecticide activities (Cohen-Inbar and Zaaroor 2016; Pucca et al. 2016b; Bordon et al. 2020; Oliveira et al. 2021; Rincon-Cortes et al. 2022). Bellow, we show another eight important and promising peptides derived from scorpion’s venom:
-
(1)
Margatoxin (MgTX) or Potassium channel toxin alpha-KTx 2.2 is a peptide of Centruroides margaritatus venom (bark scorpion, Fig. 6B). In in vivo mouse studies, MgTX leads to tumor volume reduction and blocks Kv1.3 channels. The toxin is now considered a new pharmacological prototype in the therapy of pulmonary adenocarcinoma (Jang et al. 2011).
-
(2)
The Vm24 peptide (potassium channel toxin alpha-KTx 23.1) was isolated from the venom of Vaejovis mexicanus smithi (Mexican Scorpion). Reduces the synthesis of cytokines (T cell) in response to the activation of T cell receptors (Veytia-Bucheli et al. 2018) (Fig. 1).
-
(3)
BmkTa (sodium toxin peptide) is a CTx-like peptide isolated from the venom of Mesobuthus martensii (China Scorpion). BmkTa efficiently inhibits the growth of human glioma cells (Fu et al. 2007).
-
(4)
Kaliotoxin (Ktx) or potassium channel toxin alpha-KTx 3.1 is a peptide isolated from the venom of Androctonus mauretanicus mauretanicus (Mauritanian scorpion, Fig. 6C) and is capable of preventing bone loss through osteoclastogenesis of periodontal disease, in rat models. Ktx-based drugs could be promising candidates for the treatment of periodontal disease and rheumatoid arthritis (Valverde et al. 2004).
-
(5)
ImKTX88 potassium channel toxin is a peptide toxin that was isolated from the venom of Isometrus maculatus (lesser brown scorpion). The peptide improves the pathological severity (in vivo) of autoimmune encephalomyelitis (Huang et al. 2017).
-
(6)
HsTX1 (potassium channel toxin alpha-KTx 6.3) is a peptide isolated from the venom of Heterometrus spinifer (Asian forest scorpion) with proven ability to reduce (in vivo) inflammation and arthritis (Tanner et al. 2017).
-
(7)
OSK1 (potassium channel toxin alpha-KTx 3.7) was isolated from Orthochirus scrobilosus venom (Central Asian Scorpion). This peptide can block Kv1.3, but (in vivo) OSK1 was shown to be neurotoxic in the mouse brain (Mouhat et al. 2005; Veytia-Bucheli et al. 2018).
-
(8)
The Ts6 and Ts15 peptides (potassium channel toxin alpha-KTx 12.1 and alpha-KTx 21.1, respectively) were isolated from the venom of Tityus serrulatus (Brazilian yellow scorpion). Both peptides inhibit the proliferation of memory effector T cells and reduce inflammation in a mouse model (Pucca et al. 2016a).
Bees
Bees are winged insects known for their role in pollination and, in the case of western bee species, honey production. There are more than 16,000 known bee species. Contrary to the common thought that all bees live in colonies, most species live in solitude. Species such as bumblebees and stingless bees live in colonies, while others such as stone bees, carpenter bees, leafcutter bees, and sweat bees are solitary insects (Danforth et al. 2006; Carpena et al. 2020; Khalil et al. 2021). Bee venom expresses enzymes such as phospholipase A2, peptides, and other components: biogenic amines, sugars, and minerals (Park et al. 2018; Khalil et al. 2021; Siigur and Siigur 2022; Soltan-Alinejad et al. 2022).
Melittin and Apamin are two peptides present in bee venom of Apis mellifera (Figs. 1, 012A) (Aufschnaiter et al. 2020; El-Seedi et al. 2020; Badawi 2021; Khalil et al. 2021). Melittin (Mlt) (Fig. 11A) is a peptide with 26 residues that weights 40% to 60% of the total bee venom (Yaacoub et al. 2022). Mlt contains hydrophilic and hydrophobic properties; a C-terminal with positively charged amino acids and a N-terminal of hydrophobic character. These characteristics are related to significant antibacterial and antitumor properties (Table 4) (Shi et al. 2022; Soares-Silva et al. 2022; Tang et al. 2022; Wang et al. 2022; Xie et al. 2022).
Apamin (Apm) is a peptide with 18 amino acids present in 2% to 3% of dry weight of bee venom (Gu et al. 2020). Apm has a disulfide bond that shapes its highly stable and compact chemical structure (Nguyen and Lee 2021). The application of Apm has demonstrated potential benefits in antiatherosclerosis, antiheart failure, and in the improvement of neurological disorders (Fig. 1) (Aufschnaiter et al. 2020; Abd El-Wahed et al. 2021; Gwon et al. 2021; Kuzmenkov et al. 2022).
Like other animals, bee venom has also shown anticancer activities (Aufschnaiter et al. 2020; Badawi 2021; El-Seedi et al. 2022). Preclinical and clinical studies have shown that both Mtl and Apm can regulate the cell cycle, inhibit proliferation and migration, promote apoptosis and autophagy, or even change the permeability of the cell membrane (Tables 4, 5) (Aufschnaiter et al. 2020; Carpena et al. 2020; Gu et al. 2020; Swiatly-Blaszkiewicz et al. 2020; Badawi 2021; El Mehdi et al. 2021; Mirzaei et al. 2021). Research on Melittin and Apamin showed the multitarget and multipathway implications of these toxins, which lead to protective responses against leukemia, lung, breast, cervical, pancreatic, gastric, colorectal, liver, prostate, and skin cancers (Aufschnaiter et al. 2020; Gu et al. 2020; Swiatly-Blaszkiewicz et al. 2020; Badawi 2021). In addition, the peptides are neuroprotective against multiple sclerosis and dementia (Aufschnaiter et al. 2020; Dantas et al. 2022), and exhibit anti-inflammatory (chronic skin inflammatory disease) (Dutta et al. 2019; El Mehdi et al. 2021), analgesic (Neuropathic pain) (Ye et al. 2016; Frangieh et al. 2019; Kim 2021; Kim and Kang 2022)and anti-infectivity effects (antiviral, antibacterial, antifungal effects) (El-Seedi et al. 2020, 2022; Kim 2021). Furthermore, they also showed an effect on arthritis (osteoarthritis, psoriatic arthritis, and inflammatory arthritis) (Tang et al. 2021, 2022), wound healing (Kurek-Gorecka et al. 2020a, 2020b)and antiangiogenic properties (suppression of the formation of new vascular tubes).
Amphibians
Amphibians are ectothermic vertebrates that mainly live in terrestrial, arboreal or freshwater aquatic environments. Although they have lungs, amphibians use the skin as a secondary respiratory system. The peptides stored in the granular glands of the amphibian skin are released, at high concentrations, when amphibians are stressed or injured (Varga et al. 2018). Therefore, it is not surprising that the most important amphibian toxins studied to date are those found on their skin. Amphibian peptides are mainly cationic and amphoteric, and display antimicrobial, antioxidant, antiviral and antitumor characteristics (He et al. 2013; Grayfer and Robert 2016; Casciaro et al. 2020; Feng et al. 2021a; Soltaninejad et al. 2021; Chianese et al. 2022).
Bombinin was the first short antimicrobial peptide identified (17–20 residues) (Fig. 8). It was isolated from the amphibian skin of Bombina variegate. The antimicrobial bombinin family is divided into three categories: bombinins, bombinin-like peptides (BLPs) and bombinin-H (Fig. 1). Bombinins are effective against Gram-positive and Gram-negative bacteria, as well as fungi, but they proved to be ineffective in hemolysis tests (Peng et al. 2018; Patocka et al. 2019). BLPs (BLP-1, BLP-2, and BLP-3) have high homology with other antimicrobial peptides and were isolated from Bombina orientalis skin secretions (Fig. 7A). Bombinin-H was also isolated from Bombina orientalis skin secretions and can be found in Bombina maxima secretions (Mangoni et al. 2000a; Simmaco et al. 2009; Peng et al. 2018). BHL-bombinin and Bombinin-BO1 are two members of the Bombinin family that show antibacterial activity against S. aureus and E. coli. However, Bombinin-H has little antibacterial activity, and showed very toxic effects because it promotes erythrocyte lyses (Xiang et al. 2017; Peng et al. 2018).
Esculentins are circular antimicrobial peptides isolated from the skin secretions of two frogs: the North American Rana esculenta, which secretes esculentin-1; and the Asian frog Rana ridibunda, which produces esculentin-2. Esculentin-1 is a 46 amino acid peptide with antimicrobial activity against a variety of pathogenic fungi and bacteria such as C. albicans, E. coli, S. aureus and P. aeruginosa. Although, Esculetin-2 is a smaller peptide relative to Esculentin-1, this peptide contains 37 residues that also show antimicrobial activity against E. coli, S. aureus, and C. albicans (Wang et al. 2010; Patocka et al. 2019; Casciaro et al. 2020; Malik et al. 2021).
Like Esculentins, Brevinin-1 (24 amino acids) and brevinin-2 (33 amino acids) are two antibacterial peptides isolated from the Rana brevipoda porsa. The primary and secondary structures of Brevinin-1 family peptides are conserved. Studies suggest that the proline residue at the 14th position plays a key role in the formation of a transmembrane pore, which in turn is essential for its antimicrobial activity against E. coli and S. aureus. In contrast, Brevinin-2 family peptides have at an equivalent position a Ser residue. Moreover, the C-terminal of this peptide family has a “Rana-box” disulfide motif—CKxxKxC—where x can be any amino acid and the cysteines are always in close proximity with a cationic residue, a characteristic of antimicrobial frog toxins. Brevinin-2 has a strong antibacterial activity against E. coli and S. aureus and antifungal activity against C. albicans (Savelyeva et al. 2014; Patocka et al. 2019; Jiang et al. 2020).
Ranatuerin-1 is a peptide isolated from the skin secretions of the Rana catesbeiana. Its primary sequence has 25 amino acids and forms a circular domain composed of 7 amino acids at the C-terminus. Ranatuerin-1 has strong antimicrobial activity and can inhibit the Streptococcus Group B, P. aeruginosa, S. aureus, E. coli and C. albicans (Sonnevend et al. 2004). Because of its broad-spectrum activity, it has been proposed to be an antibiotic candidate. In line, the Ranatuerin-2 peptide (ca. 30 amino acids), isolated from the skin of Rana aurora aurora, has high bacteriostatic activity against Gram-positive and Gram-negative bacteria and, as a great advantage, low hemolytic activity (Halverson et al. 2000). Ranatuerin-2 also has antimicrobial activities against: E. coli, P. aeruginosa, S. aureus, and the group B Streptococcus, like the Ranatuerin-1, but it also showed activity against K. pneumoniae, E. cloaca and S. epidermidis (Conlon et al. 2009; Zhou et al. 2019).
Ranacyclines are a family of short antimicrobial peptides (ca. 13 residues) that form a disulfide bridge between the Cys3 and Cys13, responsible for their cyclic form (Conlon et al. 2009; He et al. 2013). Both Ranacycline-B-RN1 and Ranacycline-B-RN2 have been isolated from Hylarana Nigrovittata skin and have specific antimicrobial effects against S. aureus. Two other members of this family with antimicrobial activity are Ranacycline E and Ranacycline T, isolated from Rana temporario and Rana esculenta, respectively (Patocka et al. 2019; Wang et al. 2020b). The latest have a potent antibacterial activity against Y. pseudotuberculosis YP III, B. megaterium Bm11, S. lentus, M. luteus, C. tropicalis, and C. guiller-mondii (Mangoni et al. 2003; Conlon et al. 2009; Wang et al. 2020b).
Temporins (and their derivates) are the smallest α-helical amphipathic antimicrobial peptides (10 to 14 amino acids). These peptides were isolated from several skin secretions of frogs: Rana esculenta, Rana temporaria linnaeus, Rana pipiens, Rana grylio (Fig. 7B), Rana tsushimensis, and Hylarana guentheri (Mangoni 2006). The molecular diversity of the temporins peptide family determines its functional diversity. Some temporins, like the temporin L showed antimicrobial activity against S. aureus and B. subtilis, and anticonvulsants and antitumor properties (Mangoni et al. 2000b; Wade et al. 2000; Romero et al. 2020; Jiang et al. 2021). Other molecules, such as Temporin-1Ta, Temporin-1 Tb, and Temporin-1Tl are active against Gram-positive and Gram-negative bacteria. Temporin-1Ta and temporin-1 Tb are widely used because of their low hemolytic activity. Moreover, temporin-1Tl has high hemolytic and cytotoxic activities, and thus a low therapeutic index (Bhattacharjya and Straus 2020; Wei et al. 2020; Soltaninejad et al. 2021).
Dybowskins are short peptides isolated from Rana dybowskii skin secretions (Fig. 7C). Dybowskin-1 and Dybowskin-2 have high sequence similarity and differ only by two amino acids located at positions 7 and 14. On the other hand, Dybowskin-3 to Dybowskin-6 have different sizes and low conserved sequences (Kim et al. 2007c). Dybowskins-1 to -6 showed antimicrobial activity against Gram-positive and Gram-negative bacteria such as S. aureus, B. subtilis, S. epidermidis, S. dysenteriae, M. luteus, E. coli, K. pneumoniae and the fungi C. albicans. Dybowskin-1CDYa (13 residues) and Dybowskin-2 CDYa (18 residues) display only 5% of sequence identity and low sequence similarity to known antimicrobial peptides. Yet, both peptides have strong antimicrobial activity (E. coli and S. aureus strains) but little effect on the hemolysis of human erythrocytes.
Peptides in the skin secretions of amphibians vary in structure and function. Therefore, it is not surprising that amphibians skin secretions activities are not limited to their antimicrobial role, and such peptides can be applied to other therapeutics of interest. In the following paragraphs, we briefly comment on amphibian peptides potential and how their development could be of great use in combating a wide variety of human pathologies.
Bombesin, a 14 amino acid peptide, was isolated from the skin of the Bombina bombina frog (European fire-bellied toad) (Fig. 8A) and showed a high affinity for gastrin-releasing peptide receptors (GRPR) (Tornesello et al. 2017b; Utkin 2019, 2021). Because GRPR is overexpressed in several human neoplasms, such as prostate, breast, and small cell lung cancer, the receptor could be a good target to inhibit tumors, and hence Bombesin, a potential drug candidate inspiration (Table 5) (Schwartsmann et al. 2002). Since Bombesin showed high toxicity, the peptide cannot be used in its native form; thus, scientists have tried chemical modifications to produce a stable synthetic Bombesin and increase its binding affinity and agonist/antagonist role (Cescato et al. 2008; Tornesello et al. 2015, 2017a). All bombesin-related peptides have a conserved C-terminal region characterized by the WAXGXXM-NH2 motif, but differ on N-terminal regions.
A Bombesin natural peptide isolated from the poison of the Rana bombina bombina. On the top is shown the secondary structure of the peptide, here represented as cartoon and surface. The primary sequence of the peptide is shown under this figure. Each amino acid is colored according to its polarity: grey was used for hydrophobic residues, polar residues are in cyan, special amino acids, such as glycines and prolines, are colored green, cysteines in yellow, and positive and negatively charged residues in blue and red, respectively. Non-natural amino acids are shown in squares (Alphafold model AF-P86994-F1). B Synthetic Bombesin/GRPR antagonist. In yellow/orange are highlighted the chemical modifications that transformed the natural Bombesin into the synthetic RC-3095 compound (PubChem CID 11948463)
To our knowledge, the only synthetic Bombesin/GRPR antagonist (RC-3095) evaluated to date showed tumor regressions (in vitro and in vivo) in murine and human tumor models. RC-3095 contains the bombesin residues 6 to 14, however, the N and C terminals suffered chemical modifications, resulting in the [D-Tpi6, Leu13 psi (CH2NH)-Leu14] bombesin-(6–14), as can be observed in Fig. 8B. Unfortunately, during phase I clinical trials, RC-3095 showed injection site toxicity, in patients with advanced solid malignancies, so its doses for phase II trials could not be defined (Schwartsmann et al. 2006; Umana et al. 2013; Baratto et al. 2018).
Antioxidant-rich peptides aroused scientific interest because of their ability to scavenge free radicals in the body and maintain homeostasis. Several peptides with antioxidant properties have been isolated from the skin secretions of several amphibians such as Rana plyuraden, Odorrana andersonii, Odorrana schmacker (Fig. 12F), and Odorrana lívida (Weber et al. 2005; Hwang and Daar 2017; Yang et al. 2022a). Additionally, OS-LL11 and OAVI12 peptides were isolated from the skin secretion of Odorrana schmackeri and Odorrana andersonii, respectively. Both directly reduce reactive oxygen species (ROS) levels (Calgarotto et al. 2007; Yin et al. 2019; Xie et al. 2021).
Bradykinin is a class of peptides in the kallikrein-bradykinin system (Fig. 1). Bradykinins are involved in regulation of blood pressure and preventing cardiac failure. More than 10 bradykinins have been found in amphibian skin secretions, but more studies are necessary to elucidate on their pharmacological potential (Pinheiro-Junior et al. 2018; Arichi et al. 2019; Hillmeister and Bondke Persson 2020; Gouda and Megarbane 2021; Siigur and Siigur 2022).
Other important peptides isolated from Rana palustris and Bombina variegate skin are insulin-releasing peptides (Marenah et al. 2004; Abdel-Wahab et al. 2008; Manzo et al. 2015; Conlon et al. 2020), which regulate insulin secretion in vivo and hence the blood glucose levels. Tigerinin-1R, isolated from Hoplobatrachus rugulosus (Asian frog) (Fig. 1), is a potent non-toxic insulin-releasing peptide. It has demonstrated potential for the development of new therapeutic agents against type 2 diabetes mellitus. Another peptide, Pseudin-2, stimulates insulin secretion from BRIN-BD11 cells through a mechanism that involves the Ca2+-independent pathway. [Lys18]-pseudin-2 was identified as a peptide with the potential to develop valuable insulin drugs for the treatment of type 2 diabetes (Song et al. 2009; Srinivasan et al. 2014).
Some antimicrobial peptides from amphibian’s skin secretion also showed activity to promote insulin-releasing peptides, as it is the case of Plasticin-L1 from Leptodactylus laticeps (Fig. 7D). Furthermore, strong anticarcinogenic peptides were also isolated from amphibian skin secretions. Among those we highlight the Dermaseptin-PS1 (Phyllomedusa sauvagei) and Aurein (Litoria aureus).
Along, the frog peptides Ranatensin, Litorin, and Phyllolitorin have shown activity in smooth muscle, constrict blood vessels, inhibit urine production, and are effective in the treatment of neurological diseases (Johnson et al. 1999; Cline et al. 2010; Laskowska et al. 2022).
Leeches
Leeches (Hirudinea family) are parasitic or predatory worms living in freshwater, marine, or terrestrial habitats. For centuries, mankind has used different species of leeches to draw blood from patients and to treat join diseases, extremity vein diseases, fever and rheumatic pain (Van De Car et al. 2010).
The discovery that leeches (Hiduro medicinalis) secrete a peptide called Hirudin, which prevents blood clotting, led the pharmaceutical industry to invest huge efforts in the development of thrombosis-inhibiting drugs (Fig. 9A). However, the native structure of hirudin secreted in the saliva of H. medicinalis native chemical structure resulted not to be efficient and safe for human use. However, Hirudin inspired the synthesis of two synthetic derivatives: Desirudin and Bivalirudin (Fig. 1, Fig. 9B and Fig. 9C), both acting as direct thrombin inhibitor drugs (Carswell and Plosker 2002; Warkentin and Koster 2005).
Leeches venom toxins and their derivative drugs. A Hirudin—a natural peptide isolated from the poison of Hiduro medicinalis (represented in green surface) is bound to Prothrombin (purple surface) (PDB. ID 7A0D). B 2D-representation of the chemical structure of Bivalirudin, here represented in ball and sticks (Pubchem CID 16129704). Under this figure is showed its primary sequence (spheres) and the non-natural amino acids are marked in square. The color scheme used for the amino acids follows the same pattern as showed in Fig. 8. C Desirudin (Pubchem CID 16129703)
Recombinant Desirudin (Table 1) is the active principle of Iprivask® and Revasc®, used in the clinic for vein thrombosis prophylaxis. However, for commercial reasons, they were withdrawn from the market (Kong et al. 2014). Bivalirudin, a 20 amino acid peptide, led to Angiomax® and Angiox® (Table 1) (Moen et al. 2005; Gargiulo et al. 2018). In 2000, the FDA approved the use of Angiomax® as a direct thrombin inhibitor, indicated for patients with percutaneous transluminal coronary angioplasty and unstable angina (Scholle and White 2010). Angiox® was used to prevent blood clot formation in cases of percutaneous coronary intervention and myocardial infarction, in adult patients. The last was withdrawn from the market and is no longer in use in the European Union also because of commercial reasons (Scholle and White 2010).
Lizards
The Gila monster (Heloderma suspectum) is a lizard whose bite is very painful and potentially fatal to humans. Its venomous saliva has several peptides with different functions, but exendin-4 is of particular interest (Table 1; Fig. 1). The primary sequence of exendin-4 shares about 53% of sequence identity with the glucagon-like peptide-1 receptor (Dhungat 2017; Lee et al. 2018; Coulter-Parkhill et al. 2021). The drug acts as a receptor agonist, promoting insulin secretion, and is used in the treatment of diabetes mellitus type 2, regulating insulin and glucagon levels in humans (Zhang et al. 2018b; Gentilella et al. 2019; Silva et al. 2019b; Graham et al. 2020; Coulter-Parkhill et al. 2021).
Exenatide (Byetta®) is another synthetic peptide of 39 amino acids, used for the treatment of the same type of diabetes, endorsed by the FDA (2005) and by the EMA (2006) (Table 1; Figs. 1, 11B). Exenatide demonstrated long-lasting effects in the treatment of Parkinson’s disease (Athauda and Foltynie 2018; Yang et al. 2022b), while lixisenatide showed neuroprotective effects in Alzheimer’s disease (McClean and Holscher 2014; Park et al. 2021).
Chemical modifications on exendin-4, which removed a proline and added six lysine residues, resulted in a potent 44-amino acid peptide drug—Lixisenatide (Adlyxin®) (Table 1; Fig. 1). The drug was approved by the EMA in 2013 and, later in 2016, by the FDA (Whyte et al. 2019; Graham et al. 2020; Candido et al. 2022).
Fish
Like other animals, fish venoms are also mixtures of proteins and peptides that interact with physiological processes of preys/predators causing immobilization, tissue’s digestion, and death (Church and Hodgson 2002). In 2006, Sri Balasubashini and colleagues purified a peptide—FV peptide— from the venom of the red lionfish (Pterois volitans, Fig. 10B) that showed interesting antitumor effects (Fig. 1) (Sri Balasubashini et al. 2006a). The authors proved that the peptide decreased tumor volume and the number of viable Ehrlich ascites carcinoma (ECA) cells. The exact anticancer mechanism of the FV peptide is not yet understood, but two hypotheses were suggested: 1) FV can act directly on the lytic action of the peptide; or 2) FV peptide can act indirectly, inducing apoptosis and DNA fragmentation by caspase activation in EAC (Sri Balasubashini et al. 2006b).
On the top are shown two marine animals: A the sea snail—Conus magus (left) —and B the fish—Pterois volitans (on the right), which led to the discovery of the ω-conopeptide and the FV peptide, respectively. On the bottom, C we show the northern short-tailed shrew—Blarina brevicauda—from which the Blarina toxin was identified, and, D the Giant African land snail—Lissachatina fulica—that showed ability to produce bone cements. Photos available at CalPhotos (https://calphotos.berkeley.edu). The authorship and ownership of the photographs in indicated under each photo
The SA-HT peptide is a hemorrhagic and nociceptive peptide cation-dependent peptide derived from the venom of Scatophagus argus Linn. The SA-HT toxin down-regulates the production of histamine, serotonin, cyclooxygenase, and lipoxygenase. These effects suggest that SA-HT interact with neurotransmitters, prostaglandins, and leukotrienes. Therefore, the toxin is of great interest for producing neurological or even anti-inflammatory drugs (Karmakar et al. 2004; Lopes-Ferreira et al. 2021).
Shrews
Shrews are venomous mammals found in northeastern North America. The submaxillary and sublingual glands of the Northern Short-tailed shrew (Blarina brevicauda, Fig. 10C) produce venomous saliva containing two very potent toxins (Bowen et al. 2013; Tanabe et al. 2022). A large toxin—Blarina—a 282 residues serine protease, capable of paralyzing its prey and killing small animals through paralysis of the respiratory center; and a small peptide of 54 residues, named Soricidin, that inhibits sodium channels and blocks nerve impulses in the prey (Fig. 1) (Brant and Orti 2002; Bowen et al. 2013). The peptide can inhibit the transient receptor potential vanilloid type 6 calcium channel (TRPV6). Because TRPV6 is overexpressed in a variety of cancers, Soricidin and its derivatives may be promising anticancer drug candidates (Bowen et al. 2013; Stewart 2020). In fact, SOR-C13®, a synthetic 13 amino acid peptide derived from the C-terminal region of Soricidin, suggested antitumor activity, leading to a reduction in solid pancreatic tumor (up to 27%) (Fu et al. 2017). So far, SOR-C13 has not revealed serious drug-related adverse events, and the FDA included the peptide in phase I clinical trial for the treatment of ovarian and pancreatic cancers (Table 5), (Fu et al. 2017).
Snails
Snail venoms, both marine and terrestrial, have a large amount and variety of attractive bioactive compounds for the cosmetic and pharmaceutical industries (Dhiman and Pant 2021). Their toxins can be useful in producing new formulations with reduced toxicity and side effects compared to current drugs (Lebbe et al. 2014).
Crude extracts, mucus and sludge contain many bioactive peptides which have been used, in traditional medicine, against viral lesions, warts and other dermatologic problems, pain, microbial infections, and tumors (Table 6) (Akondi et al. 2014; Dhiman and Pant 2021; Salimi et al. 2021).
Venoms from many species of cone snails (Conus ssp.) contain small cysteine-rich peptides (10 to 30 residues) (see Table 6). These peptides are associated with the Conotoxin family (CNTx) (Lewis 2009; Li et al. 2021b; Tran et al. 2022; Wiere et al. 2022) (Table 6 and Fig. 1). In particular, the ω-conopeptide isolated from the venom of the magic cone snail (Conus magus, Fig. 10A) is the most notable and famous peptide example from the Conotoxin family (Kristipati et al. 1994; Olivera et al. 1994; Favreau et al. 1999; Fedosov et al. 2013; Eisapoor et al. 2016; Chalil et al. 2021). This peptide was the inspiration to produce the synthetic ω-conotoxin MVIIA, named Ziconotide (Fig. 11C). It is a small cyclic peptide of 25 amino acids and 3 intramolecular disulfide bonds, with high affinity for N-type voltage-gated calcium channel. The drug was the first calcium channel inhibitor derived from venom with clinical application. It inhibits the release of nociceptive neurochemicals, resulting in severe chronic pain relief (Lugo-Fabres et al. 2021; Trevisan and Oliveira 2022; Wie and Derian 2022). This analgesic drug was first approved by the FDA (2004) and then by the EMA (2005), for severe chronic pain, which was until theses dates considered intractable, and is commercialized under the trade name Prialt® (Table 1), (Rigo et al. 2013; Loschner et al. 2021; Jergova et al. 2022; Wie and Derian 2022). Despite being 3 orders of magnitude more potent than morphine, it has low efficacy when administered orally and intravenously. To maintain its analgesic potential, the drug must be administered intrathecally, i.e. directly into the spinal fluid, which limits its regular use (Pope and Deer 2013; Deer et al. 2019; Matis et al. 2021; Sorrieul et al. 2021; Holden et al. 2022) (Fig. 11).
Other venom peptides inspired the design of three approved drugs. (A) Apitox ® derived from melittin, a peptide from Bee venom used against knee and arthrosis pain (PDB ID. 2MLT) (Eisenberg et al. 1990)(B) The Gila monster derivative originated a drug used against type II diabetes due to its high similarity to the glucagon-like peptide Byetta® (PDB ID. 6GDZ) (Evers et al. 2018). C The ω-conotoxin of the cone snail led to ziconotide, an approved drug for pain treatment, commercialized under the trade name Prialt®. The human N-type voltage gated calcium channel CaV2.2 -alpha2/delta1-beta1 (represented in blue surface) is bound to ziconotide (in pink) (PDB ID. 7VFU) (Dong et al. 2021). The complex receptor-drug-POPC bilayer membrane (stick representation) was assembled using the Charmm-Gui webserver (Jo et al. 2008). Figures were created with BioRender.com (2020) (https://app.biorender.com/biorender-templates). Under each secondary structure figure is showed its primary sequence (spheres) and the. non-natural amino acids are marked in square. The color scheme used for the amino acids follows the same pattern as showed in Figs. 8 and 9
Animal whose venoms led to drugs in preclinical phase. The insects: A Western honey bee (Apis mellifera) and B Waps from the Vespula genus produce the Mellitin toxin and the Mastoparan peptide, respectively. C Spiders from the genus Lycosa such as the wolf spider (Lycosa erythrognatha) inspired the synthetic peptide LyeTxI-b. D The banked krait cobra (Bungarus fasciatus) inspired the design of the LZ1 peptide. E Centipedes from the Scolopendra genus, in particular, the Chinese red-headed centipede (Scolopendra subspinipes mutilans) produce the µ-SLPTX-Ssm6a toxin. F The Schmacker's frog (Odorrana schmackeri) secretes antioxidant peptides (OS-LLs). Photos available at CalPhotos (https://calphotos.berkeley.edu). The authorship and ownership of the photographs in indicated under each photo
Perspective
Natural products and their derivatives have always contributed to a great extent to new approved drugs. Noble examples of their importance are the antibiotics, in particular, the penicillin obtained from Penicillium moulds. Today, about one-third of the FDA-approved drugs are natural products. Almost half of these are derived from mammals, one-quarter from plants, and one-quarter from microbes (Patridge et al. 2016).
From the chemical point of view, natural products are complex biomolecules that cannot be explored by small-drug-like high-throughput virtual screening techniques. However, combinatorial chemistry techniques have succeeded in optimizing structures and have been extensively used in the transformation of many recently approved natural agents, improving drug stability and oral bioavailability. Since the 1930s, natural products have diminished, and synthetic and semisynthetic natural product derivatives have increased.
Despite the efforts of the scientific community to find new drugs, there are still big gaps in the treatment of diseases such as the neurodegenerative and cancer pathologies, among others. To date, there are no effective drugs that can reduce or rescue neurodegenerative debilitating symptoms or that can act as effective anticancer therapies, overcoming the cytotoxic effects of current chemotherapies. Moreover, there is an emergent need to develop new antibiotics against several resistant bacteria strains or new antiviral therapies (Kinch et al. 2014).
Natural products derived from animal venoms have been used in traditional medicine for eras. Despite lacking scientific explanation, they have been a cure for many years in traditional societies (Charitos et al. 2022). Recent advances in sequencing technologies, proteomic, structural, and analytical techniques not only explain theories, beliefs, and experiences, where alternative medicines are based, but now shed light on the molecular determinants that assist their pharmacological activities (Simoes-Silva et al. 2018). This breakthrough opens the use of venoms as a therapeutic and diagnostic source (Almeida et al. 2017). Allowing, the exploitation of a complete novel chemical space never studied before.
Animal venoms are truly near-unexplored mines of bioactive compounds. A common feature of animal venom is the presence of active peptides that can be used for biological purposes (Almeida et al. 2017). A characteristic of peptide toxins is that these molecules are highly selective and potent binders for a wide range of molecular targets and are associated with important pharmacological activities (Riciluca et al. 2021; Cao et al. 2022). A sustainable discovery strategy associated with biotechnology has allowed bioactive compounds derived from animal venoms, particularly peptide toxins, to stand out in the development of new biopharmaceuticals applied to therapy and diagnosis.
The drug discovery process is a long-term risk challenge that requires huge financial investments without a guarantee of success. This is a relevant fact regarding peptides derived from animal venoms (terrestrial and marine), which always require structural changes both to avoid their toxic effects and to obtain more efficient molecules. Although venoms are relevant sources of bioactive components for new leading molecules and powerful tools for the investigation of transmembrane receptors, ion channels, and enzymes, only a few of these toxins have reached the market. Captopril®, Integrilin®, Apitox®, Byetta®, and Prialt® are some successful examples of the use of animal peptides as lead compounds used in the development of new pharmaceuticals and medical therapies (other successful examples are shown in Table 1). Currently, many drugs based on toxins from different animals are being analyzed in preclinical (Table 2) or clinical phases (Table 3). As we saw in this review, worldwide interests, both scientific and business, are producing many drugs based on animal peptides, mainly due to the potential of their medical applications and bioactivities (Boldrini-Franca et al. 2017). The literature still shows how little we know about the jumbo biodiversity of the planet, from the biological, ecological, pharmacological, medicinal, and biotechnological points of view. Many more molecules, such as those discussed here, can be exploited sustainably, consolidating the bioeconomy. However, with the accelerated extinction species rate resources, we are losing many species without even knowing them. Those venoms might be among the endless sources of therapeutics that can alleviate the diseases afflicting humankind.
Availability of data and material
Not applicable.
References
Abbade LPF, Barraviera S, Silvares MRC et al (2021) Treatment of chronic venous ulcers with heterologous fibrin sealant: a phase I/II clinical trial. Front Immunol. https://doi.org/10.3389/fimmu.2021.627541
Abd El-Wahed A, Yosri N, Sakr HH et al (2021) Wasp venom biochemical components and their potential in biological applications and nanotechnological interventions. Toxins (basel). https://doi.org/10.3390/toxins13030206
Abdel-Salam MAL, Carvalho-Tavares J, Gomes KS et al (2019) The synthetic peptide LyeTxI-b derived from Lycosa erythrognatha spider venom is cytotoxic to U-87 MG glioblastoma cells. Amino Acids 51(3):433–449. https://doi.org/10.1007/s00726-018-2678-4
Abdel-Wahab YH, Power GJ, Ng MT et al (2008) Insulin-releasing properties of the frog skin peptide pseudin-2 and its [Lys18]-substituted analogue. Biol Chem 389(2):143–148. https://doi.org/10.1515/BC.2008.018
Akef HM (2018) Anticancer, antimicrobial, and analgesic activities of spider venoms. Toxicol Res (camb) 7(3):381–395. https://doi.org/10.1039/c8tx00022k
Akondi KB, Muttenthaler M, Dutertre S et al (2014) Discovery, synthesis, and structure-activity relationships of conotoxins. Chem Rev 114(11):5815–5847. https://doi.org/10.1021/cr400401e
Almeida, J.R.; Lancellotti, M.; Soares, A.M. et al (2016). CoaTx-II, a new dimeric Lys49 phospholipase A2 from Crotalus oreganus abyssus snake venom with bactericidal potential: Insights into its structure and biological roles. Toxicon. 120(147–158. doi:: https://doi.org/10.1016/j.toxicon.2016.08.007
Almeida JR, Mendes B, Lancellotti M et al (2018) A novel synthetic peptide inspired on Lys49 phospholipase A(2) from Crotalus oreganus abyssus snake venom active against multidrug-resistant clinical isolates. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2018.02.055
Almeida JR, Palacios ALV, Patino RSP et al (2019) Harnessing snake venom phospholipases A2 to novel approaches for overcoming antibiotic resistance. Drug Dev Res 80(1):68–85. https://doi.org/10.1002/ddr.21456
Almeida JR, Resende LM, Watanabe RK et al (2017) Snake Venom Peptides and Low Mass Proteins: Molecular Tools and Therapeutic Agents. Curr Med Chem 24(30):3254–3282. https://doi.org/10.2174/0929867323666161028155611
Arichi S, Sasaki-Hamada S, Kadoya Y et al (2019) Excitatory effect of bradykinin on intrinsic neurons of the rat heart. Neuropeptides. https://doi.org/10.1016/j.npep.2019.04.002
Athauda D, Foltynie T (2018) Protective effects of the GLP-1 mimetic exendin-4 in Parkinson’s disease. Neuropharmacology 136(Pt B):260–270. https://doi.org/10.1016/j.neuropharm.2017.09.023
Aufschnaiter A, Kohler V, Khalifa S et al (2020) Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: limitations and possibilities. Toxins (basel). https://doi.org/10.3390/toxins12020066
Bachmann M, Kukkurainen S, Hytonen VP et al (2019) Cell adhesion by integrins. Physiol Rev 99(4):1655–1699. https://doi.org/10.1152/physrev.00036.2018
Badawi JK (2021) Bee venom components as therapeutic tools against prostate cancer. Toxins (basel). https://doi.org/10.3390/toxins13050337
Bagheri-Ziari S, Shahbazzadeh D, Sardari S et al (2021) Discovery of a new analgesic peptide, leptucin, from the Iranian Scorpion, Hemiscorpius lepturus. Molecules. https://doi.org/10.3390/molecules26092580
Baratto L, Jadvar H, Iagaru A (2018) Prostate cancer theranostics targeting gastrin-releasing peptide receptors. Mol Imaging Biol 20(4):501–509. https://doi.org/10.1007/s11307-017-1151-1
Basnyat B, Shilpakar O (2022) Snakebite envenoming: a hidden health crisis. Lancet Glob Health 10(3):311–312. https://doi.org/10.1016/S2214-109X(22)00029-8
Ben-Mabrouk H, Zouari-Kessentini R, Montassar F et al (2016) CC5 and CC8, two homologous disintegrins from Cerastes cerastes venom, inhibit in vitro and ex vivo angiogenesis. Int J Biol Macromol 86:670–680. https://doi.org/10.1016/j.ijbiomac.2016.02.008
Berde CB, Athiraman U, Yahalom B et al (2011) Tetrodotoxin-bupivacaine-epinephrine combinations for prolonged local anesthesia. Mar Drugs 9(12):2717–2728. https://doi.org/10.3390/md9122717
Bhattacharjya S, Straus SK (2020) Design, engineering and discovery of novel alpha-helical and beta-boomerang antimicrobial peptides against drug resistant bacteria. Int J Mol Sci 21(16):5773. https://doi.org/10.3390/ijms21165773
Boldrini-Franca J, Cologna CT, Pucca MB et al (2017) Minor snake venom proteins: Structure, function and potential applications. Biochim Biophys Acta Gen Subj 1861(4):824–838. https://doi.org/10.1016/j.bbagen.2016.12.022
Boldrini-Franca J, Pinheiro-Junior EL, Arantes EC (2019) Functional and biological insights of rCollinein-1, a recombinant serine protease from Crotalus durissus collilineatus. J Venom Anim Toxins Incl Trop Dis 25:147118. https://doi.org/10.1590/1678-9199-JVATITD-1471-18
Boldrini-Franca J, Pinheiro-Junior EL, Peigneur S et al (2020) Beyond hemostasis: a snake venom serine protease with potassium channel blocking and potential antitumor activities. Sci Rep 10(1):4476. https://doi.org/10.1038/s41598-020-61258-x
Bond A (2006) Exenatide (Byetta) as a novel treatment option for type 2 diabetes mellitus. Proc (bayl Univ Med Cent) 19(3):281–284. https://doi.org/10.1080/08998280.2006.11928181
Bordon KCF, Cologna CT, Fornari-Baldo EC et al (2020) From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front Pharmacol 11:1132. https://doi.org/10.3389/fphar.2020.01132
Bowen CV, DeBay D, Ewart HS et al (2013) In vivo detection of human TRPV6-rich tumors with anti-cancer peptides derived from soricidin. PLoS ONE 8(3):58866. https://doi.org/10.1371/journal.pone.0058866
Brant SV, Orti G (2002) Molecular phylogeny of short-tailed shrews, Blarina (Insectivora: Soricidae). Mol Phylogenet Evol 22(2):163–173. https://doi.org/10.1006/mpev.2001.1057
Bressan E, Touska F, Vetter I et al (2016) Crotalphine desensitizes TRPA1 ion channels to alleviate inflammatory hyperalgesia. Pain 157(11):2504–2516. https://doi.org/10.1097/j.pain.0000000000000669
Brigatte P, Konno K, Gutierrez VP et al (2013) Peripheral kappa and delta opioid receptors are involved in the antinociceptive effect of crotalphine in a rat model of cancer pain. Pharmacol Biochem Behav. https://doi.org/10.1016/j.pbb.2013.04.012
Brutsch DR, Hunziker P, Pot S et al (2020) Corneal and scleral permeability of Desmoteplase in different species. Vet Ophthalmol 23(5):785–791. https://doi.org/10.1111/vop.12782
Bucciarelli GM, Lechner M, Fontes A et al (2021) From poison to promise: The evolution of tetrodotoxin and its potential as a therapeutic. Toxins (basel). https://doi.org/10.3390/toxins13080517
Buchaim DV, Cassaro CV, Shindo J et al (2019) Unique heterologous fibrin biopolymer with hemostatic, adhesive, sealant, scaffold and drug delivery properties: a systematic review. J Venom Anim Toxins Incl Trop Dis. https://doi.org/10.1590/1678-9199-JVATITD-2019-0038
Butte PV, Mamelak A, Parrish-Novak J et al (2014) Near-infrared imaging of brain tumors using the Tumor Paint BLZ-100 to achieve near-complete resection of brain tumors. Neurosurg Focus 36(2):1. https://doi.org/10.3171/2013.11.FOCUS13497
Calderon LA, Sobrinho JC, Zaqueo KD et al (2014) Antitumoral activity of snake venom proteins: new trends in cancer therapy. Biomed Res Int. 2014:20639. https://doi.org/10.1155/2014/203639
Calgarotto AK, Miotto S, Honório KM et al (2007) A multivariate study on flavonoid compounds scavenging the peroxynitrite free radical. J Mol Struct 808:25–33
Calvete JJ (2018) Snake venomics—from low-resolution toxin-pattern recognition to toxin-resolved venom proteomes with absolute quantification. Expert Rev Proteomics 15(7):555–568. https://doi.org/10.1080/14789450.2018.1500904
Calvete JJ, Fox JW, Agelan A et al (2002) The presence of the WGD motif in CC8 heterodimeric disintegrin increases its inhibitory effect on alphaII(b)beta3, alpha(v)beta3, and alpha5beta1 integrins. Biochemistry 41(6):2014–2021. https://doi.org/10.1021/bi015627o
Calvete JJ, Jurgens M, Marcinkiewicz C et al (2000) Disulphide-bond pattern and molecular modelling of the dimeric disintegrin EMF-10, a potent and selective integrin alpha5beta1 antagonist from Eristocophis macmahoni venom. Biochem J. 345:573–581
Calvete JJ, McLane MA, Stewart GJ et al (1994) Characterization of the cross-linking site of disintegrins albolabrin, bitistatin, echistatin, and eristostatin on isolated human platelet integrin GPIIb/IIIa. Biochem Biophys Res Commun 202(1):135–140. https://doi.org/10.1006/bbrc.1994.1903
Calvete JJ, Wang Y, Mann K et al (1992) The disulfide bridge pattern of snake venom disintegrins, flavoridin and echistatin. FEBS Lett 309(3):316–320. https://doi.org/10.1016/0014-5793(92)80797-k
Camargo AC, Ianzer D, Guerreiro JR et al (2012) Bradykinin-potentiating peptides: beyond captopril. Toxicon 59(4):516–523. https://doi.org/10.1016/j.toxicon.2011.07.013
Candido R, Modugno M, Larosa M et al (2022) Effectiveness, safety, and appropriateness in the use of the fixed-ratio combination of insulin glargine and lixisenatide in type 2 diabetes: the ENSURE retrospective real-world study. Diabetes Ther. https://doi.org/10.1007/s13300-022-01328-7
Cao Z, Shahbazzadeh D, Kovacic H et al (2022) Editorial: venoms, animal and microbial toxins, volume II. Front Pharmacol 13:973628. https://doi.org/10.3389/fphar.2022.973628
Cardoso BG, de Lima WG, Fernandes SOA et al (2022) Antifungal activity of a shortened analogue of the natural peptide LyeTx I isolated from the venom of the spider Lycosa erythrognatha. Nat Prod Res. https://doi.org/10.1080/14786419.2022.2079122
Cardoso FC, Dekan Z, Smith JJ et al (2017) Modulatory features of the novel spider toxin mu-TRTX-Df1a isolated from the venom of the spider Davus fasciatus. Br J Pharmacol 174(15):2528–2544. https://doi.org/10.1111/bph.13865
Carpena M, Nunez-Estevez B, Soria-Lopez A et al (2020) Bee venom: an updating review of its bioactive molecules and its health applications. Nutrients 12:11. https://doi.org/10.3390/nu12113360
Carswell CI, Plosker GL (2002) Bivalirudin: a review of its potential place in the management of acute coronary syndromes. Drugs 62(5):841–870. https://doi.org/10.2165/00003495-200262050-00008
Casciaro B, Cappiello F, Loffredo MR et al (2020) The potential of frog skin peptides for anti-infective therapies: the case of esculentin-1a(1–21)NH2. Curr Med Chem 27(9):1405–1419. https://doi.org/10.2174/0929867326666190722095408
Cesar PHS, Braga MA, Trento MVC et al (2019) Snake venom disintegrins: an overview of their interaction with integrins. Curr Drug Targets 20(4):465–477. https://doi.org/10.2174/1389450119666181022154737
Cescato R, Maina T, Nock B et al (2008) Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med 49(2):318–326. https://doi.org/10.2967/jnumed.107.045054
Chalil A, Staudt MD, Harland TA et al (2021) A safety review of approved intrathecal analgesics for chronic pain management. Expert Opin Drug Saf 20(4):439–451. https://doi.org/10.1080/14740338.2021.1889513
Chan YW, Tan KY, Tan CH (2022) Preclinical assessment of VPEAV, a new trivalent antivenom for elapid snakebite envenoming in the Philippines: proteomics, immunoreactivity and toxicity neutralization. Toxicon 220:106942. https://doi.org/10.1016/j.toxicon.2022.106942
Charitos IA, Gagliano-Candela R, Santacroce L et al (2022) Venoms and poisonings during the centuries: a narrative review. Endocr Metab Immune Disord Drug Targets 22(6):558–570. https://doi.org/10.2174/1871530320666200904105816
Chassagnon IR, McCarthy CA, Chin YK et al (2017) Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc Natl Acad Sci USA 114(14):3750–3755. https://doi.org/10.1073/pnas.1614728114
Chen Y, Wang Y, Zhai Y et al (2023) Cinobufacini injection suppresses the proliferation of human osteosarcoma cells by inhibiting PIN1-YAP/TAZ signaling pathway. Front Pharmacol 14:1081363. https://doi.org/10.3389/fphar.2023.1081363
Chen YC, Chang YT, Chen CY et al (2020) Structural insight into integrin recognition and anticancer activity of echistatin. Toxins (basel) 12:11. https://doi.org/10.3390/toxins12110709
Chianese A, Zannella C, Monti A et al (2022) The broad-spectrum antiviral potential of the amphibian peptide AR-23. Int J Mol Sci 23:2. https://doi.org/10.3390/ijms23020883
Chiang CY, Lin CY, Chen YT et al (2016) Blue fluorescent protein derived from the mutated purple chromoprotein isolated from the sea anemone Stichodactyla haddoni. Protein Eng Des Sel 29(11):523–530. https://doi.org/10.1093/protein/gzw041
Chiang HS, Swaim MW, Huang TF (1994) Characterization of platelet aggregation induced by human colon adenocarcinoma cells and its inhibition by snake venom peptides, trigramin and rhodostomin. Br J Haematol 87(2):325–331. https://doi.org/10.1111/j.1365-2141.1994.tb04917.x
Chiang HS, Swaim MW, Huang TF (1995) Characterization of platelet aggregation induced by human breast carcinoma and its inhibition by snake venom peptides, trigramin and rhodostomin. Breast Cancer Res Treat 33(3):225–235. https://doi.org/10.1007/BF00665947
Chiou JT, Wang LJ, Lee YC et al (2021) Naja atra cardiotoxin 1 induces the FasL/Fas death pathway in human leukemia cells. Cells. 10:8. https://doi.org/10.3390/cells10082073
Chippaux JP (2008) Estimating the global burden of snakebite can help to improve management. PLoS Med 5(11):221. https://doi.org/10.1371/journal.pmed.0050221
Chippaux JP, Massougbodji A, Habib AG (2019) The WHO strategy for prevention and control of snakebite envenoming: a sub-Saharan Africa plan. J Venom Anim Toxins Incl Trop Dis. https://doi.org/10.1590/1678-9199-JVATITD-2019-0083
Christensen M, Knop FK, Holst JJ et al (2009) Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. Drugs. 12(8):503–513
Church JE, Hodgson WC (2002) The pharmacological activity of fish venoms. Toxicon 40(8):1083–1093. https://doi.org/10.1016/s0041-0101(02)00126-5
Cline MA, Cofield SA, Tachibana T (2010) Central litorin injection is associated with primary anorexigenic effects that coincide with activation of the magnocellular division of the paraventricular nucleus. Neuropeptides 44(3):247–252. https://doi.org/10.1016/j.npep.2009.12.015
Cohen-Inbar O, Zaaroor M (2016) Glioblastoma multiforme targeted therapy: the Chlorotoxin story. J Clin Neurosci 33:52–58. https://doi.org/10.1016/j.jocn.2016.04.012
Conlon JM, Attoub S, Musale V et al (2020) Isolation and characterization of cytotoxic and insulin-releasing components from the venom of the black-necked spitting cobra Naja nigricollis (Elapidae). Toxicon X 6:100030. https://doi.org/10.1016/j.toxcx.2020.100030
Conlon JM, Kolodziejek J, Nowotny N (2009) Antimicrobial peptides from the skins of North American frogs. Biochim Biophys Acta 1788(8):1556–1563. https://doi.org/10.1016/j.bbamem.2008.09.018
Correa EA, Kayano AM, Diniz-Sousa R et al (2016) Isolation, structural and functional characterization of a new Lys49 phospholipase A2 homologue from Bothrops neuwiedi urutu with bactericidal potential. Toxicon 115:13–21. https://doi.org/10.1016/j.toxicon.2016.02.021
Coulter-Parkhill A, McClean S, Gault VA et al (2021) Therapeutic potential of peptides derived from animal venoms: current views and emerging drugs for diabetes. Clin Med Insights Endocrinol Diabetes 14:11795514211006072. https://doi.org/10.1177/11795514211006071
Czarnomysy R, Surazynski A, Poplawska B et al (2017) Synergistic action of cisplatin and echistatin in MDA-MB-231 breast cancer cells. Mol Cell Biochem 427(1–2):13–22. https://doi.org/10.1007/s11010-016-2894-8
da Silva Caldeira CA, Diniz-Sousa R, Pimenta DC et al (2021) Antimicrobial peptidomes of Bothrops atrox and Bothrops jararacussu snake venoms. Amino Acids 53(10):1635–1648. https://doi.org/10.1007/s00726-021-03055-y
da Silva JF, Castro-Junior CJ, Oliveira SM et al (2015) Characterization of the antinociceptive effect of PhTx3-4, a toxin from Phoneutria nigriventer, in models of thermal, chemical and incisional pain in mice. Toxicon 108:53–61. https://doi.org/10.1016/j.toxicon.2015.09.043
Daidone I, Aschi M, Patamia M et al (2013) Structural and dynamical properties of KTS-disintegrins: a comparison between Obtustatin and Lebestatin. Biopolymers 99(1):47–54. https://doi.org/10.1002/bip.22138
Danen EH, Marcinkiewicz C, Cornelissen IM et al (1998) The disintegrin eristostatin interferes with integrin alpha 4 beta 1 function and with experimental metastasis of human melanoma cells. Exp Cell Res 238(1):188–196. https://doi.org/10.1006/excr.1997.3821
Danforth BN, Sipes S, Fang J et al (2006) The history of early bee diversification based on five genes plus morphology. Proc Natl Acad Sci USA 103(41):15118–15123. https://doi.org/10.1073/pnas.0604033103
Dantas CG, da Paixao AO, Nunes T et al (2022) Africanized bee venom (Apis mellifera Linnaeus): neuroprotective effects in a Parkinson’s disease mouse model induced by 6-hydroxydopamine. Toxics. https://doi.org/10.3390/toxics10100583
Dardevet L, Najlaoui F, Aroui S et al (2022) A Conjugate between Lqh-8/6, a natural peptide analogue of chlorotoxin, and doxorubicin efficiently induces glioma cell death. Biomedicines 10:10. https://doi.org/10.3390/biomedicines10102605
Dash TS, Shafee T, Harvey PJ et al (2019) A centipede toxin family defines an ancient class of CSalphabeta defensins. Structure 27(2):315–326. https://doi.org/10.1016/j.str.2018.10.022
de Avelar Junior JT, Lima-Batista E, Castro Junior CJ et al (2022) LyeTxI-b, a synthetic peptide derived from a spider venom, is highly active in triple-negative breast cancer cells and acts synergistically with cisplatin. Front Mol Biosci 9:876833. https://doi.org/10.3389/fmolb.2022.876833
Deer TR, Pope JE, Hanes MC et al (2019) Intrathecal therapy for chronic pain: a review of morphine and ziconotide as firstline options. Pain Med 20(4):784–798. https://doi.org/10.1093/pm/pny132
Delinois LJ, Peon H, Villalobos-Santos JC et al (2020) A cytochrome c-chlorotoxin hybrid protein as a possible antiglioma drug. ChemMedChem 15(22):2185–2192. https://doi.org/10.1002/cmdc.202000373
Dhiman V, Pant D (2021) Human health and snails. J Immunoassay Immunochem 42(3):211–235. https://doi.org/10.1080/15321819.2020.1844751
Dhungat JP (2017) Gila monster lizard & incretin-mimetics. J Assoc Phys India 65(4):96
Diego-Garcia E, Peigneur S, Waelkens E et al (2010) Venom components from Citharischius crawshayi spider (Family Theraphosidae): exploring transcriptome, venomics, and function. Cell Mol Life Sci 67(16):2799–2813. https://doi.org/10.1007/s00018-010-0359-x
Diniz-Sousa R, Caldeira CAS, Kayano AM et al (2018) Identification of the molecular determinants of the antibacterial activity of LmutTX, a Lys49 phospholipase A2 homologue isolated from Lachesis muta muta snake venom (Linnaeus, 1766). Basic Clin Pharmacol Toxicol 122(4):413–423. https://doi.org/10.1111/bcpt.12921
Diniz-Sousa R, Silva CCA, Pereira SS et al (2023) Therapeutic applications of snake venoms: An invaluable potential of new drug candidates. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2023.124357
Dodd RA, Cornwell R, Holm NE et al (2002) The Vivostat application system: a comparison with conventional fibrin sealant application systems. Technol Health Care 10(5):401–411
Dong Y, Gao Y, Xu S et al (2021) Closed-state inactivation and pore-blocker modulation mechanisms of human Ca(V)2.2. Cell Rep 37(5):109931. https://doi.org/10.1016/j.celrep.2021.109931
Dutta P, Sahu RK, Dey T et al (2019) Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem Biol Interact 313:108824. https://doi.org/10.1016/j.cbi.2019.108824
Eisapoor SS, Jamili S, Shahbazzadeh D et al (2016) A new, high yield, rapid, and cost-effective protocol to deprotection of cysteine-rich conopeptide, omega-conotoxin MVIIA. Chem Biol Drug Des 87(5):687–693. https://doi.org/10.1111/cbdd.12702
Eisenberg D, Gribskov M, Terwilliger TC (1990) Melittin.
El-Seedi H, Abd El-Wahed A, Yosri N et al (2020) Antimicrobial properties of Apis mellifera’s Bee Venom. Toxins (basel) 12:7. https://doi.org/10.3390/toxins12070451
El-Seedi HR, Yosri N, El-Aarag B et al (2022) Chemistry and the potential antiviral, anticancer, and anti-inflammatory activities of cardiotonic steroids derived from toads. Molecules 27:19. https://doi.org/10.3390/molecules27196586
El Mehdi I, Falcao SI, Harandou M et al (2021) Chemical, cytotoxic, and anti-inflammatory assessment of honey bee venom from Apis mellifera intermissa. Antibiotics (basel) 10:12. https://doi.org/10.3390/antibiotics10121514
Ellison M, Feng ZP, Park AJ et al (2008) Alpha-RgIA, a novel conotoxin that blocks the alpha9alpha10 nAChR: structure and identification of key receptor-binding residues. J Mol Biol 377(4):1216–1227. https://doi.org/10.1016/j.jmb.2008.01.082
Ellison M, Haberlandt C, Gomez-Casati ME et al (2006) Alpha-RgIA: a novel conotoxin that specifically and potently blocks the alpha9alpha10 nAChR. Biochemistry 45(5):1511–1517. https://doi.org/10.1021/bi0520129
Elmaraezy A, Abushouk AI, Saad S et al (2017) Desmoteplase for acute ischemic stroke: a systematic review and metaanalysis of randomized controlled trials. CNS Neurol Disord Drug Targets 16(7):789–799. https://doi.org/10.2174/1871527315666161213110104
Emerich BL, Ferreira RCM, Machado-de-Avila RA et al (2020) PnAn13, an antinociceptive synthetic peptide inspired in the Phoneutria nigriventer toxin PnTx4(6–1) (delta-Ctenitoxin-Pn1a). Toxicon X 7:100045. https://doi.org/10.1016/j.toxcx.2020.100045
Estevao-Costa MI, Sanz-Soler R, Johanningmeier B et al (2018) Snake venom components in medicine: From the symbolic rod of Asclepius to tangible medical research and application. Int J Biochem Cell Biol 104:94–113. https://doi.org/10.1016/j.biocel.2018.09.011
Esteves FG, Dos Santos-Pinto JRA, Ferro M et al (2020) Revealing the venomous secrets of the spider’s web. J Proteome Res 19(8):3044–3059. https://doi.org/10.1021/acs.jproteome.0c00086
Estrada G, Villegas E, Corzo G (2007) Spider venoms: a rich source of acylpolyamines and peptides as new leads for CNS drugs. Nat Prod Rep 24(1):145–161. https://doi.org/10.1039/b603083c
Evers A, Bossart M, Pfeiffer-Marek S et al (2018) Dual glucagon-like peptide 1 (GLP-1)/glucagon receptor agonists specifically optimized for multidose formulations. J Med Chem 61(13):5580–5593. https://doi.org/10.1021/acs.jmedchem.8b00292
Fainzilber M, Gordon D, Hasson A et al (1991) Mollusc-specific toxins from the venom of Conus textile neovicarius. Eur J Biochem 202(2):589–595. https://doi.org/10.1111/j.1432-1033.1991.tb16412.x
Fang Y, He X, Zhang P et al (2019) In vitro and in vivo antimalarial activity of LZ1, a peptide derived from snake cathelicidin. Toxins (Basel) 11:7. https://doi.org/10.3390/toxins11070379
Favreau P, Le Gall F, Benoit E et al (1999) A review on conotoxins targeting ion channels and acetylcholine receptors of the vertebrate neuromuscular junction. Acta Physiol Pharmacol Ther Latinoam 49(4):257–267
Fedosov AE, Moshkovskii SA, Kuznetsova KG et al (2013) Conotoxins: from the biodiversity of gastropods to new drugs. Biomed Khim 59(3):267–294. https://doi.org/10.18097/pbmc20135903267
Feng G, Wu J, Yang HL et al (2021) Discovery of antioxidant peptides from amphibians: a review. Protein Pept Lett. 28(11):1220–1229. https://doi.org/10.2174/0929866528666210907145634
Feng W, Wang W, Meng R et al (2021b) Lixisenatide is effective and safe as add-on treatment to basal insulin in Asian individuals with type 2 diabetes and different body mass indices: a pooled analysis of data from the GetGoal Studies. BMJ Open Diabetes Res Care 9:1. https://doi.org/10.1136/bmjdrc-2021-002290
Fernandez-Rojo MA, Deplazes E, Pineda SS et al (2018) Gomesin peptides prevent proliferation and lead to the cell death of devil facial tumour disease cells. Cell Death Discov 4:19. https://doi.org/10.1038/s41420-018-0030-0
Ferreira RS Jr, de Barros LC, Abbade LPF et al (2017) Heterologous fibrin sealant derived from snake venom: from bench to bedside—an overview. J Venom Anim Toxins Incl Trop Dis 23:21. https://doi.org/10.1186/s40409-017-0109-8
Fidel J, Kennedy KC, Dernell WS et al (2015) Preclinical validation of the utility of BLZ-100 in providing fluorescence contrast for imaging spontaneous solid tumors. Cancer Res 75(20):4283–4291. https://doi.org/10.1158/0008-5472.CAN-15-0471
Fischer T, Riedl R (2022) Paracelsus’ legacy in the faunal realm: drugs deriving from animal toxins. Drug Discov Today 27(2):567–575. https://doi.org/10.1016/j.drudis.2021.10.003
Fisher R, Hassenbusch S, Krames E et al (2005) A consensus statement regarding the present suggested titration for prialt (ziconotide). Neuromodulation 8(3):153–154. https://doi.org/10.1111/j.1525-1403.2005.05232.x
Flannery L (2007) Diabetes quiz. How much do you know about Byetta? Diabetes Self Manag. 24(4):52, 54
Frangieh J, Rima M, Fajloun Z et al (2021) Snake venom components: tools and cures to target cardiovascular diseases. Molecules 26:8. https://doi.org/10.3390/molecules26082223
Frangieh J, Salma Y, Haddad K et al (2019) First characterization of the venom from Apis mellifera syriaca, a honeybee from the Middle East Region. Toxins (basel) 11:4. https://doi.org/10.3390/toxins11040191
Frauz K, Teodoro LFR, Carneiro GD et al (2019) Transected tendon treated with a new fibrin sealant alone or associated with adipose-derived stem cells. Cells 8:1. https://doi.org/10.3390/cells8010056
Fu S, Hirte H, Welch S et al (2017) First-in-human phase I study of SOR-C13, a TRPV6 calcium channel inhibitor, in patients with advanced solid tumors. Invest New Drugs 35(3):324–333. https://doi.org/10.1007/s10637-017-0438-z
Fu YJ, Yin LT, Liang AH et al (2007) Therapeutic potential of chlorotoxin-like neurotoxin from the Chinese scorpion for human gliomas. Neurosci Lett 412(1):62–67. https://doi.org/10.1016/j.neulet.2006.10.056
Gan ZR, Gould RJ, Jacobs JW et al (1988) Echistati.n A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. J Biol Chem. 263(36):19827–19832
Gandomkari MS, Ayat H, Ahadi AM (2022) Recombinantly expressed MeICT, a new toxin from Mesobuthus eupeus scorpion, inhibits glioma cell proliferation and downregulates Annexin A2 and FOXM1 genes. Biotechnol Lett 44(5–6):703–712. https://doi.org/10.1007/s10529-022-03254-x
Gao S, Yao X, Yan N (2021) Structure of human Ca(v)22 channel blocked by the painkiller ziconotide. Nature 596(7870):143–147. https://doi.org/10.1038/s41586-021-03699-6
Gargiulo G, Carrara G, Frigoli E et al (2018) Bivalirudin or heparin in patients undergoing invasive management of acute coronary syndromes. J Am Coll Cardiol 71(11):1231–1242. https://doi.org/10.1016/j.jacc.2018.01.033
Garsky VM, Lumma PK, Freidinger RM et al (1989) Chemical synthesis of echistatin, a potent inhibitor of platelet aggregation from Echis carinatus: synthesis and biological activity of selected analogs. Proc Natl Acad Sci USA 86(11):4022–4026. https://doi.org/10.1073/pnas.86.11.4022
Gentilella R, Pechtner V, Corcos A et al (2019) Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: are they all the same? Diabetes Metab Res Rev 35(1):3070. https://doi.org/10.1002/dmrr.3070
Ghazy E, Taghi HS (2022) The autophagy-inducing mechanisms of vitexin, cinobufacini, and physalis alkekengi hydroalcoholic extract against breast cancer in vitro and in vivo. J Gastrointest Cancer 53(3):592–596. https://doi.org/10.1007/s12029-021-00668-0
Giardini AC, Evangelista BG, Sant’Anna MB et al (2021) Crotalphine attenuates pain and neuroinflammation induced by experimental autoimmune encephalomyelitis in mice. Toxins (Basel) 13:11. https://doi.org/10.3390/toxins13110827
Goldlust SA, Kavoosi M, Nezzer J et al (2021) Tetrodotoxin for chemotherapy-induced neuropathic pain: a randomized, double-blind, placebo-controlled, parallel-dose finding trial. Toxins (Basel) 13:4. https://doi.org/10.3390/toxins13040235
Gomena J, Vari B, Olah-Szabo R et al (2023) Targeting the gastrin-releasing peptide receptor (GRP-R) in cancer therapy: development of bombesin-based peptide-drug conjugates. Int J Mol Sci 24:4. https://doi.org/10.3390/ijms24043400
Gomez RS, Casali TA, Romano-Silva MA et al (1995) The effect of PhTx3 on the release of 3H-acetylcholine induced by tityustoxin and potassium in brain cortical slices and myenteric plexus. Neurosci Lett 196(1–2):131–133. https://doi.org/10.1016/0304-3940(95)11843-l
Goncalves-Machado L, Pla D, Sanz L et al (2016) Combined venomics, venom gland transcriptomics, bioactivities, and antivenomics of two Bothrops jararaca populations from geographic isolated regions within the Brazilian Atlantic rainforest. J Proteomics 135:73–89. https://doi.org/10.1016/j.jprot.2015.04.029
Goncalves TC, Benoit E, Kurz M et al (2019a) From identification to functional characterization of cyriotoxin-1a, an antinociceptive toxin from the spider Cyriopagopus schioedtei. Br J Pharmacol 176(9):1298–1314. https://doi.org/10.1111/bph.14628
Goncalves TC, Lesport P, Kuylle S et al (2019b) Evaluation of the spider (Phlogiellus genus) phlotoxin 1 and synthetic variants as antinociceptive drug candidates. Toxins (basel) 11:9. https://doi.org/10.3390/toxins11090484
Goncaves JM, Ferreira J, Prado MA et al (2011) The effect of spider toxin PhTx3-4, omega-conotoxins MVIIA and MVIIC on glutamate uptake and on capsaicin-induced glutamate release and [Ca2+]i in spinal cord synaptosomes. Cell Mol Neurobiol 31(2):277–283. https://doi.org/10.1007/s10571-010-9618-5
Gonzalez-Cano R, Ruiz-Cantero MC, Santos-Caballero M et al (2021) Tetrodotoxin, a potential drug for neuropathic and cancer pain relief? Toxins (basel) 13:7. https://doi.org/10.3390/toxins13070483
Gouda AS, Megarbane B (2021) Snake venom-derived bradykinin-potentiating peptides: a promising therapy for COVID-19? Drug Dev Res 82(1):38–48. https://doi.org/10.1002/ddr.21732
Graham GV, McLaughlin CM, Flatt PR (2020) Role of exendin-4 in the Gila monster: Further lessons regarding human oral glucagon-like peptide-1 therapy? Diabetes Obes Metab 22(12):2509–2511. https://doi.org/10.1111/dom.14171
Gray WR, Luque A, Olivera BM et al (1981) Peptide toxins from Conus geographus venom. J Biol Chem 256(10):4734–4740
Grayfer L, Robert J (2016) Amphibian macrophage development and antiviral defenses. Dev Comp Immunol 58:60–67. https://doi.org/10.1016/j.dci.2015.12.008
Gu H, Han SM, Park KK (2020) Therapeutic effects of apamin as a bee venom component for non-neoplastic disease. Toxins (Basel) 12:3. https://doi.org/10.3390/toxins12030195
Guimaraes CL, Moreira-Dill LS, Fernandes RS et al (2014) Biodiversity as a source of bioactive compounds against snakebites. Curr Med Chem 21(25):2952–2979. https://doi.org/10.2174/09298673113206660295
Gutierrez VP, Konno K, Chacur M et al (2008) Crotalphine induces potent antinociception in neuropathic pain by acting at peripheral opioid receptors. Eur J Pharmacol 594(1–3):84–92. https://doi.org/10.1016/j.ejphar.2008.07.053
Gwon MG, An HJ, Gu H et al (2021) Apamin inhibits renal fibrosis via suppressing TGF-beta1 and STAT3 signaling in vivo and in vitro. J Mol Med (Berl) 99(9):1265–1277. https://doi.org/10.1007/s00109-021-02087-x
Hailey S, Adams E, Penn R et al (2013) Effect of the disintegrin eristostatin on melanoma-natural killer cell interactions. Toxicon 61:83–93. https://doi.org/10.1016/j.toxicon.2012.10.011
Halverson T, Basir YJ, Knoop FC et al (2000) Purification and characterization of antimicrobial peptides from the skin of the North American green frog Rana clamitans. Peptides 21(4):469–476. https://doi.org/10.1016/s0196-9781(00)00178-9
Harrington RA (1997) Design and methodology of the PURSUIT trial: evaluating eptifibatide for acute ischemic coronary syndromes. Platelet Glycoprotein IIb-IIIa in Unstable Angina: receptor suppression using integrilin therapy. Am J Cardiol 80(4):34–38. https://doi.org/10.1016/s0002-9149(97)00575-4
Harris C, Smith GH (1981) Captopril (Capoten, E.R. Squibb & Sons). Drug Intell Clin Pharm 15(12):932–939. https://doi.org/10.1177/106002808101501203
Harvey AL (2014) Toxins and drug discovery. Toxicon 92:193–200. https://doi.org/10.1016/j.toxicon.2014.10.020
Hayashi MAF, Campeiro JD, Yonamine CM (2022) Revisiting the potential of South American rattlesnake Crotalus durissus terrificus toxins as therapeutic, theranostic and/or biotechnological agents. Toxicon 206:1–13. https://doi.org/10.1016/j.toxicon.2021.12.005
He X, Yang S, Wei L et al (2013) Antimicrobial peptide diversity in the skin of the torrent frog, Amolops Jingdongensis. Amino Acids 44(2):481–487. https://doi.org/10.1007/s00726-012-1358-z
Herzig V, Cristofori-Armstrong B, Israel MR et al (2020) Animal toxins - Nature’s evolutionary-refined toolkit for basic research and drug discovery. Biochem Pharmacol 181:114096. https://doi.org/10.1016/j.bcp.2020.114096
Hillmeister P, Bondke Persson A (2020) Bradykinin-from snake poison to therapeutic options. Acta Physiol (Oxf) 228(3):13445. https://doi.org/10.1111/apha.13445
Holden R, Chauhan G, Emerick T (2022) Intrathecal administration of ziconotide as a potential treatment for chronic migraines. Cureus 14(3):23714. https://doi.org/10.7759/cureus.23714
Hong SY, Koh YS, Chung KH et al (2002) Snake venom disintegrin, saxatilin, inhibits platelet aggregation, human umbilical vein endothelial cell proliferation, and smooth muscle cell migration. Thromb Res 105(1):79–86. https://doi.org/10.1016/s0049-3848(01)00416-9
Huang CY, Cheng YM, Li W et al (2023) Examining the mechanisms of huachansu injection on liver cancer through integrated bioinformatics analysis. Recent Pat Anticancer Drug Discov 18(3):408–425. https://doi.org/10.2174/1574892817666220511162046
Huang J, Han S, Sun Q et al (2017) Kv1.3 channel blocker (ImKTx88) maintains blood-brain barrier in experimental autoimmune encephalomyelitis. Cell Biosci 7:31. https://doi.org/10.1186/s13578-017-0158-2
Huang TF, Holt JC, Lukasiewicz H et al (1987) Trigramin. A low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb-IIIa complex. J Biol Chem 262(33):16157–16163
Huang TF, Peng HC, Peng IS et al (1992) An antiplatelet peptide, gabonin, from Bitis gabonica snake venom. Arch Biochem Biophys 298(1):13–20. https://doi.org/10.1016/0003-9861(92)90087-d
Huang Y, Ng XW, Lim SG et al (2016) In vivo evaluation of cenderitide-eluting stent (CES) II. Ann Biomed Eng 44(2):432–441. https://doi.org/10.1007/s10439-015-1389-1
Huang ZG, Lv FM, Wang J et al (2019) RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability. Int J Pharm 556:217–225. https://doi.org/10.1016/j.ijpharm.2018.12.023
Huynh TT, van Dam EM, Sreekumar S et al (2022) Copper-67-labeled bombesin peptide for targeted radionuclide therapy of prostate cancer. Pharmaceuticals (Basel) 15:6. https://doi.org/10.3390/ph15060728
Hwang YS, Daar IO (2017) A frog’s view of EphrinB signaling. Genesis 55:1–2. https://doi.org/10.1002/dvg.23002
Ichiki T, Dzhoyashvili N, Burnett JC Jr (2019) Natriuretic peptide based therapeutics for heart failure: Cenderitide: a novel first-in-class designer natriuretic peptide. Int J Cardiol 281:166–171. https://doi.org/10.1016/j.ijcard.2018.06.002
Ikonomopoulou MP, Fernandez-Rojo MA, Pineda SS et al (2018) Gomesin inhibits melanoma growth by manipulating key signaling cascades that control cell death and proliferation. Sci Rep 8(1):11519. https://doi.org/10.1038/s41598-018-29826-4
Isbister GK, Gray MR, Balit CR et al (2005) Funnel-web spider bite: a systematic review of recorded clinical cases. Med J Aust 182(8):407–411. https://doi.org/10.5694/j.1326-5377.2005.tb06760.x
Jacoby DB, Dyskin E, Yalcin M et al (2010) Potent pleiotropic anti-angiogenic effects of TM601, a synthetic chlorotoxin peptide. Anticancer Res 30(1):39–46
Jang SH, Choi SY, Ryu PD et al (2011) Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur J Pharmacol 651(1–3):26–32. https://doi.org/10.1016/j.ejphar.2010.10.066
Jang YJ, Kim DS, Jeon OH et al (2007) Saxatilin suppresses tumor-induced angiogenesis by regulating VEGF expression in NCI-H460 human lung cancer cells. J Biochem Mol Biol 40(3):439–443. https://doi.org/10.5483/bmbrep.2007.40.3.439
Jared C, Mailho-Fontana PL, Antoniazzi MM (2021) Differences between poison and venom: an attempt at an integrative biological approach. Acta Zool 102:337–350
Jergova S, Hernandez M, Sagen J (2022) Analgesic effect of recombinant GABAergic precursors releasing omega-conotoxin MVIIA in a model of peripheral nerve injury in rats. Mol Pain 18:17448069221129828. https://doi.org/10.1177/17448069221129829
Jiang X, Zhang X, Fu C et al (2021) Antineoplastic effects and mechanisms of a new RGD chimeric peptide from bullfrog skin on the proliferation and apoptosis of B16F10 cells. Protein J 40(5):709–720. https://doi.org/10.1007/s10930-021-09980-x
Jiang Y, Wu Y, Wang T et al (2020) Brevinin-1GHd: a novel Hylarana guentheri skin secretion-derived Brevinin-1 type peptide with antimicrobial and anticancer therapeutic potential. Biosci Rep 40:5
Jiang ZS, Xia CF, Tian QP et al (2000) Effect of batroxobin against dog heart ischemia/reperfusion injury. Acta Pharmacol Sin 21(1):70–74
Jo S, Kim T, Iyer VG et al (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29(11):1859–1865. https://doi.org/10.1002/jcc.20945
Johnson MD, Ko M, Choo KS et al (1999) The effects of the phyllolitorin analogue [desTrp(3), Leu(8)]phyllolitorin on scratching induced by bombesin and related peptides in rats. Brain Res 839(1):194–198. https://doi.org/10.1016/s0006-8993(99)01708-4
Jouirou B, Mosbah A, Visan V et al (2004) Cobatoxin 1 from Centruroides noxius scorpion venom: chemical synthesis, three-dimensional structure in solution, pharmacology and docking on K+ channels. Biochem J 377(Pt 1):37–49. https://doi.org/10.1042/BJ20030977
Jridi I, Catacchio I, Majdoub H et al (2015) Hemilipin, a novel Hemiscorpius lepturus venom heterodimeric phospholipase A2, which inhibits angiogenesis in vitro and in vivo. Toxicon 105:34–44. https://doi.org/10.1016/j.toxicon.2015.08.022
Jridi I, Catacchio I, Majdoub H et al (2017) The small subunit of Hemilipin2, a new heterodimeric phospholipase A2 from Hemiscorpius lepturus scorpion venom, mediates the antiangiogenic effect of the whole protein. Toxicon 126:38–46. https://doi.org/10.1016/j.toxicon.2016.12.001
Ju X, Fan D, Kong L et al (2021) Antimicrobial peptide brevinin-1RL1 from frog skin secretion induces apoptosis and necrosis of tumor cells. Molecules 26:7. https://doi.org/10.3390/molecules26072059
Jump W (1982) Drug corner: capoten (Captopril). Crit Care Nurse 2(1): 64–65, 69
Kabanova NV, Vassilevski AA, Rogachevskaja OA et al (2012) Modulation of P2X3 receptors by spider toxins. Biochim Biophys Acta 1818(11):2868–2875. https://doi.org/10.1016/j.bbamem.2012.07.016
Karmakar S, Muhuri DC, Dasgupta SC et al (2004) Isolation of a haemorrhagic protein toxin (SA-HT) from the Indian venomous butterfish (Scatophagus argus, Linn) sting extract. Indian J Exp Biol 42(5):452–460
Kashyap VS, Bishop PD, Bena JF et al (2010) A pilot, prospective evaluation of a direct thrombin inhibitor, bivalirudin (Angiomax), in patients undergoing lower extremity bypass. J Vasc Surg 52(2):369–374. https://doi.org/10.1016/j.jvs.2010.02.276
Kawakami R, Lee CYW, Scott C et al (2018) A human study to evaluate safety, tolerability, and cyclic GMP activating properties of cenderitide in subjects with stable chronic heart failure. Clin Pharmacol Ther 104(3):546–552. https://doi.org/10.1002/cpt.974
Kazemi-Lomedasht F, Khalaj V, Bagheri KP et al (2017) The first report on transcriptome analysis of the venom gland of Iranian scorpion, Hemiscorpius lepturus. Toxicon 125:123–130. https://doi.org/10.1016/j.toxicon.2016.11.261
Kerkis I, Hayashi MA, Prieto da Silva AR et al (2014) State of the art in the studies on crotamine, a cell penetrating peptide from South American rattlesnake. Biomed Res Int 2014:675985. https://doi.org/10.1155/2014/675985
Kesavan K, Ratliff J, Johnson EW et al (2010) Annexin A2 is a molecular target for TM601, a peptide with tumor-targeting and anti-angiogenic effects. J Biol Chem 285(7):4366–4374. https://doi.org/10.1074/jbc.M109.066092
Khalil A, Elesawy BH, Ali TM et al (2021) Bee venom: from venom to drug. Molecules 26:16. https://doi.org/10.3390/molecules26164941
Khamessi O, Ben Mabrouk H, Kamoun S et al (2022) The first snake venom KTS/disintegrins-integrin interactions using bioinformatics approaches. Molecules 28:1. https://doi.org/10.3390/molecules28010325
Khan S, Gul A, Noreen R et al (2018) Potential applications of venom peptides as anti-thrombotic agents for management of arterial and deep-vein thrombosis. Protein Pept Lett 25(7):677–687. https://doi.org/10.2174/0929866524666180614100101
Kilham HA, Isbister GK (2020) Australian funnel-web spider envenoming. J Paediatr Child Health 56(12):1843–1845. https://doi.org/10.1111/jpc.15134
Kim D, Kang KH (2022) Anti-inflammatory and anti-bacterial potential of mulberry leaf extract on oral microorganisms. Int J Environ Res Public Health 19:9. https://doi.org/10.3390/ijerph19094984
Kim DS, Jang YJ, Jeon OH et al (2007a) Saxatilin inhibits TNF-alpha-induced proliferation by suppressing AP-1-dependent IL-8 expression in the ovarian cancer cell line MDAH 2774. Mol Immunol 44(6):1409–1416. https://doi.org/10.1016/j.molimm.2006.05.001
Kim DS, Jang YJ, Jeon OH et al (2007b) Saxatilin, a snake venom disintegrin, suppresses TNF-alpha-induced ovarian cancer cell invasion. J Biochem Mol Biol 40(2):290–294. https://doi.org/10.5483/bmbrep.2007.40.2.290
Kim HS, Jeong HY, Lee YK et al (2013) Synergistic antitumoral effect of IL-12 gene cotransfected with antiangiogenic genes for angiostatin, endostatin, and saxatilin. Oncol Res 21(4):209–216. https://doi.org/10.3727/096504014X13907540404798
Kim SS, Shim MS, Chung J et al (2007c) Purification and characterization of antimicrobial peptides from the skin secretion of Rana dybowskii. Peptides 28(8):1532–1539. https://doi.org/10.1016/j.peptides.2007.07.002
Kim W (2021) Bee venom and its sub-components: characterization, pharmacology, and therapeutics. Toxins (Basel) 13:3. https://doi.org/10.3390/toxins13030191
Kimm T, Bean BP (2014) Inhibition of A-type potassium current by the peptide toxin SNX-482. J Neurosci 34(28):9182–9189. https://doi.org/10.1523/JNEUROSCI.0339-14.2014
Kinch MS, Patridge E, Plummer M et al (2014) An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today 19(9):1283–1287. https://doi.org/10.1016/j.drudis.2014.07.005
Kjaergard HK, Trumbull HR (1998) Vivostat system autologous fibrin sealant: preliminary study in elective coronary bypass grafting. Ann Thorac Surg 66(2):482–486. https://doi.org/10.1016/s0003-4975(98)00470-6
Knudsen KA, Tuszynski GP, Huang TF et al (1988) Trigramin, an RGD-containing peptide from snake venom, inhibits cell-substratum adhesion of human melanoma cells. Exp Cell Res 179(1):42–49. https://doi.org/10.1016/0014-4827(88)90346-1
Kohlmeier KA, Leonard CS (2006) Transmitter modulation of spike-evoked calcium transients in arousal related neurons: muscarinic inhibition of SNX-482-sensitive calcium influx. Eur J Neurosci 23(5):1151–1162. https://doi.org/10.1111/j.1460-9568.2006.04640.x
Kong Y, Chen H, Wang YQ et al (2014) Direct thrombin inhibitors: patents 2002–2012 (Review). Mol Med Rep 9(5):1506–1514. https://doi.org/10.3892/mmr.2014.2025
Konno K, Picolo G, Gutierrez VP et al (2008) Crotalphine, a novel potent analgesic peptide from the venom of the South American rattlesnake Crotalus durissus terrificus. Peptides 29(8):1293–1304. https://doi.org/10.1016/j.peptides.2008.04.003
Koppel DM (1991) Clarification of vasotec and teprotide dosages. Postgrad Med 89(8):39. https://doi.org/10.1080/00325481.1991.11700951
Kristipati R, Nadasdi L, Tarczy-Hornoch K et al (1994) Characterization of the binding of omega-conopeptides to different classes of non-L-type neuronal calcium channels. Mol Cell Neurosci 5(3):219–228. https://doi.org/10.1006/mcne.1994.1026
Kurek-Gorecka A, Gorecki M, Rzepecka-Stojko A et al (2020a) Bee products in dermatology and skin care. Molecules 25:3. https://doi.org/10.3390/molecules25030556
Kurek-Gorecka A, Komosinska-Vassev K, Rzepecka-Stojko A et al (2020) Bee venom in wound healing. Molecules 26:1. https://doi.org/10.3390/molecules26010148
Kuwada M, Teramoto T, Kumagaye KY et al (1994) Omega-agatoxin-TK containing D-serine at position 46, but not synthetic omega-[L-Ser46]agatoxin-TK, exerts blockade of P-type calcium channels in cerebellar Purkinje neurons. Mol Pharmacol 46(4):587–593
Kuzmenkov AI, Nekrasova OV, Peigneur S et al (2018) K(V)1.2 channel-specific blocker from Mesobuthus eupeus scorpion venom: structural basis of selectivity. Neuropharmacology 143:228–238. https://doi.org/10.1016/j.neuropharm.2018.09.030
Kuzmenkov AI, Peigneur S, Nasburg JA et al (2022) Apamin structure and pharmacology revisited. Front Pharmacol 13:977440. https://doi.org/10.3389/fphar.2022.977440
Lan T, Chen HF, Zheng F et al (2023) Cinobufacini retards progression of pancreatic ductal adenocarcinoma through targeting YEATS2/TAK1/NF-kappaB axis. Phytomedicine 109:154564. https://doi.org/10.1016/j.phymed.2022.154564
Laskowska AK, Szudzik M, Sciezynska A et al (2022) The role of a natural amphibian skin-based peptide, ranatensin, in pancreatic cancers expressing dopamine D2 receptors. Cancers Basel 14:22. https://doi.org/10.3390/cancers14225535
Latallo ZS, Lopaciuk S (1973) New approach to thrombolytic therapy: the use of Defibrase in connection with streptokinase. Thromb Diath Haemorrh Suppl 56:253–264
Lazarovici P, Marcinkiewicz C, Lelkes PI (2019) From snake venom’s disintegrins and C-type lectins to anti-platelet drugs. Toxins (Basel) 11:5. https://doi.org/10.3390/toxins11050303
Lebbe EK, Peigneur S, Maiti M et al (2014) Discovery of a new subclass of alpha-conotoxins in the venom of Conus australis. Toxicon 91:145–154. https://doi.org/10.1016/j.toxicon.2014.08.074
Lee CY, Huntley BK, McCormick DJ et al (2016) Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions. Eur Heart J Cardiovasc Pharmacother 2(2):98–105. https://doi.org/10.1093/ehjcvp/pvv040
Lee JG, Ryu JH, Kim SM et al (2018) Replacement of the C-terminal Trp-cage of exendin-4 with a fatty acid improves therapeutic utility. Biochem Pharmacol 151:59–68. https://doi.org/10.1016/j.bcp.2018.03.004
Levy DE, del Zoppo GJ, Demaerschalk BM et al (2009a) Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke 40(12):3796–3803. https://doi.org/10.1161/STROKEAHA.109.565119
Levy DE, Trammel J, Wasiewski WW et al (2009b) Ancrod for acute ischemic stroke: a new dosing regimen derived from analysis of prior ancrod stroke studies. J Stroke Cerebrovasc Dis 18(1):23–27. https://doi.org/10.1016/j.jstrokecerebrovasdis.2008.07.009
Lewis RJ (2009) Conotoxin venom peptide therapeutics. Adv Exp Med Biol 655:44–48. https://doi.org/10.1007/978-1-4419-1132-2_5
Li CL, Yang R, Sun Y et al (2021a) N58A exerts analgesic effect on trigeminal neuralgia by regulating the MAPK pathway and tetrodotoxin-resistant sodium channel. Toxins (basel) 13:5. https://doi.org/10.3390/toxins13050357
Li M, Shao X, Wu C et al (2020) Chlorotoxin-derived bicyclic peptides for targeted imaging of glioblastomas. Chem Commun (Camb) 56(66):9537–9540. https://doi.org/10.1039/d0cc01089h
Li ML, Liao RW, Qiu JW et al (2000) Antimicrobial activity of synthetic all-D mastoparan M. Int J Antimicrob Agents 13(3):203–208. https://doi.org/10.1016/s0924-8579(99)00127-2
Li QW, Ma LF, Liu CB et al (2022a) Cinobufacini inhibits the development of pancreatic cancer cells through the TGFbeta/smads pathway of pancreatic stellate cells. Evid Based Complement Altern Med 2022:3719857. https://doi.org/10.1155/2022/3719857
Li R, Wu H, Wang M et al (2022) An integrated strategy to delineate the chemical and dynamic metabolic profile of Huachansu tablets in rat plasma based on UPLC-ESI-QTOF/MS(E). J Pharm Biomed Anal 218:114866. https://doi.org/10.1016/j.jpba.2022.114866
Li X, Ling L, Li C et al (2017) Efficacy and safety of desmoteplase in acute ischemic stroke patients: a systematic review and meta-analysis. Medicine (Baltimore) 96(18):e6667. https://doi.org/10.1097/MD.0000000000006667
Li X, Tae HS, Chu Y et al (2021) Medicinal chemistry, pharmacology, and therapeutic potential of alpha-conotoxins antagonizing the alpha9alpha10 nicotinic acetylcholine receptor. Pharmacol Ther 222:107792. https://doi.org/10.1016/j.pharmthera.2020.107792
Lima WG, Brito JCM, de Lima ME et al (2021) A short synthetic peptide, based on LyeTx I from Lycosa erythrognatha venom, shows potential to treat pneumonia caused by carbapenem-resistant Acinetobacter baumannii without detectable resistance. J Antibiot (Tokyo) 74(7):425–434. https://doi.org/10.1038/s41429-021-00421-6
Lin CH, Shyu CL, Wu ZY et al (2023) Antimicrobial peptide mastoparan-AF kills multi-antibiotic resistant Escherichia coli O157:H7 via multiple membrane disruption patterns and likely by adopting 3–11 amphipathic helices to favor membrane interaction. Membranes (Basel) 13:2. https://doi.org/10.3390/membranes13020251
Lippens G, Najib J, Wodak SJ et al (1995) NMR sequential assignments and solution structure of chlorotoxin, a small scorpion toxin that blocks chloride channels. Biochemistry 34(1):13–21. https://doi.org/10.1021/bi00001a003
Liu B, Huang L, Xu R et al (2022) An improved isotope labelling method for quantifying deamidated cobratide using high-resolution quadrupole-orbitrap mass spectrometry. Molecules 27:19. https://doi.org/10.3390/molecules27196154
Liu B, Wang W, Gao T et al (2021) Separation, identification and quantification of associated impurities in cobratide using sheathless CE-MS and CE-UV. Anal Methods 13(34):3845–3851. https://doi.org/10.1039/d1ay00717c
Liu S, Marder VJ, Levy DE et al (2011) Ancrod and fibrin formation: perspectives on mechanisms of action. Stroke 42(11):3277–3280. https://doi.org/10.1161/STROKEAHA.111.622753
Lopes-Ferreira M, Sosa-Rosales I, Silva Junior PI et al (2021) Molecular characterization and functional analysis of the nattectin-like toxin from the venomous fish thalassophryne maculosa. Toxins (Basel) 14:1. https://doi.org/10.3390/toxins14010002
Lopes FSR, Giardini AC, Sant’Anna MB et al (2022) Crotalphine modulates microglia M1/M2 phenotypes and induces spinal analgesia mediated by opioid-cannabinoid systems. Int J Mol Sci 23:19. https://doi.org/10.3390/ijms231911571
Loschner D, Dries R, Kalff R et al (2021) What became of Prialt(R)? : observational study on the use of ziconotide in the treatment of chronic pain. Schmerz 35(5):343–348. https://doi.org/10.1007/s00482-021-00531-y
Lucena SE, Jia Y, Soto JG et al (2012) Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis). Toxicon 60(1):31–39. https://doi.org/10.1016/j.toxicon.2012.03.011
Luddecke T, Herzig V, von Reumont BM et al (2022) The biology and evolution of spider venoms. Biol Rev Camb Philos Soc 97(1):163–178. https://doi.org/10.1111/brv.12793
Lugo-Fabres PH, Otero-Sastre LM, Bernaldez-Sarabia J et al (2021) Potential therapeutic applications of synthetic conotoxin s-cal14.2b, derived from Californiconus californicus, for treating type 2 diabetes. Biomedicines 9:8. https://doi.org/10.3390/biomedicines9080936
Ma R, Mahadevappa R, Kwok HF (2017) Venom-based peptide therapy: insights into anti-cancer mechanism. Oncotarget 8(59):100908–100930. https://doi.org/10.18632/oncotarget.21740
Mackessy SP (2022) Venom production and secretion in reptiles. J Exp Biol 225:7. https://doi.org/10.1242/jeb.227348
Malik E, Phoenix DA, Snape TJ et al (2021) Linearized esculentin-2EM shows pH dependent antibacterial activity with an alkaline optimum. Mol Cell Biochem 476(10):3729–3744. https://doi.org/10.1007/s11010-021-04181-7
Mambelli-Lisboa NC, Sciani JM, Prieto B, da Silva AR et al (2018) Co-localization of crotamine with internal membranes and accentuated accumulation in tumor cells. Molecules 23:4. https://doi.org/10.3390/molecules23040968
Mangoni ML (2006) Temporins, anti-infective peptides with expanding properties. Cell Mol Life Sci 63(9):1060–1069. https://doi.org/10.1007/s00018-005-5536-y
Mangoni ML, Grovale N, Giorgi A et al (2000a) Structure-function relationships in bombinins H, antimicrobial peptides from Bombina skin secretions. Peptides 21(11):1673–1679. https://doi.org/10.1016/s0196-9781(00)00316-8
Mangoni ML, Papo N, Mignogna G et al (2003) Ranacyclins, a new family of short cyclic antimicrobial peptides: biological function, mode of action, and parameters involved in target specificity. Biochemistry 42(47):14023–14035. https://doi.org/10.1021/bi034521l
Mangoni ML, Rinaldi AC, Di Giulio A et al (2000b) Structure-function relationships of temporins, small antimicrobial peptides from amphibian skin. Eur J Biochem 267(5):1447–1454. https://doi.org/10.1046/j.1432-1327.2000.01143.x
Manzo G, Scorciapino MA, Srinivasan D et al (2015) Conformational analysis of the host-defense peptides pseudhymenochirin-1Pb and -2Pa and design of analogues with insulin-releasing activities and reduced toxicities. J Nat Prod 78(12):3041–3048. https://doi.org/10.1021/acs.jnatprod.5b00843
Marcinkiewicz C, Calvete JJ, Vijay-Kumar S et al (1999) Structural and functional characterization of EMF10, a heterodimeric disintegrin from Eristocophis macmahoni venom that selectively inhibits alpha 5 beta 1 integrin. Biochemistry 38(40):13302–13309. https://doi.org/10.1021/bi9906930
Marenah L, Flatt PR, Orr DF et al (2004) Brevinin-1 and multiple insulin-releasing peptides in the skin of the frog Rana palustris. J Endocrinol 181(2):347–354. https://doi.org/10.1677/joe.0.1810347
Matavel A, Cruz JS, Penaforte CL et al (2002) Electrophysiological characterization and molecular identification of the Phoneutria nigriventer peptide toxin PnTx2-6. FEBS Lett 523(1–3):219–223. https://doi.org/10.1016/s0014-5793(02)02988-5
Matheson AJ, Goa KL (2000) Desirudin: a review of its use in the management of thrombotic disorders. Drugs 60(3):679–700. https://doi.org/10.2165/00003495-200060030-00012
Matis G, De Negri P, Dupoiron D et al (2021) Intrathecal pain management with ziconotide: time for consensus? Brain Behav 11((suppl 1)):e2055. https://doi.org/10.1002/brb3.2055
Matthews EA, Bee LA, Stephens GJ et al (2007) The Cav2.3 calcium channel antagonist SNX-482 reduces dorsal horn neuronal responses in a rat model of chronic neuropathic pain. Eur J Neurosci 25(12):3561–3569. https://doi.org/10.1111/j.1460-9568.2007.05605.x
Mazzucco L, Balbo V, Cattana E et al (2008) Platelet-rich plasma and platelet gel preparation using Plateltex. Vox Sang 94(3):202–208. https://doi.org/10.1111/j.1423-0410.2007.01027.x
Mazzucco L, Balbo V, Cattana E et al (2009) Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang 97(2):110–118. https://doi.org/10.1111/j.1423-0410.2009.01188.x
McClean PL, Holscher C (2014) Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology 86:241–258. https://doi.org/10.1016/j.neuropharm.2014.07.015
McLane MA, Vijay-Kumar S, Marcinkiewicz C et al (1996) Importance of the structure of the RGD-containing loop in the disintegrins echistatin and eristostatin for recognition of alpha IIb beta 3 and alpha v beta 3 integrins. FEBS Lett 391(1–2):139–143. https://doi.org/10.1016/0014-5793(96)00716-8
Mellado G, Espinoza N, Garate JA et al (2022) Spider toxin SNX-482 gating modifier spontaneously partitions in the membrane guided by electrostatic interactions. Membranes (basel) 12:6. https://doi.org/10.3390/membranes12060595
Mendes B, Almeida JR, Vale N et al (2019) Potential use of 13-mer peptides based on phospholipase and oligoarginine as leishmanicidal agents. Comput Biochem Physiol C 226:8612. https://doi.org/10.1016/j.cbpc.2019.108612
Menezes C, Thakur NL (2022) Sea anemone venom: ecological interactions and bioactive potential. Toxicon 208:31–46. https://doi.org/10.1016/j.toxicon.2022.01.004
Meng P, Huang H, Wang G et al (2016) A novel toxin from Haplopelma lividum selectively inhibits the Na(V)1.8 channel and possesses potent analgesic efficacy. Toxins (Basel) 9:1. https://doi.org/10.3390/toxins9010007
Menting JG, Gajewiak J, MacRaild CA et al (2016) A minimized human insulin-receptor-binding motif revealed in a Conus geographus venom insulin. Nat Struct Mol Biol 23(10):916–920. https://doi.org/10.1038/nsmb.3292
Min CK, Lee JW, Chung KH et al (2010) Control of specific growth rate to enhance the production of a novel disintegrin, saxatilin, in recombinant Pichia pastoris. J Biosci Bioeng 110(3):314–319. https://doi.org/10.1016/j.jbiosc.2010.03.013
Minassian NA, Gibbs A, Shih AY et al (2013) Analysis of the structural and molecular basis of voltage-sensitive sodium channel inhibition by the spider toxin huwentoxin-IV (mu-TRTX-Hh2a). J Biol Chem 288(31):22707–22720. https://doi.org/10.1074/jbc.M113.461392
Mirzaei S, Fekri HS, Hashemi F et al (2021) Venom peptides in cancer therapy: An updated review on cellular and molecular aspects. Pharmacol Res 164:105327. https://doi.org/10.1016/j.phrs.2020.105327
Moen MD, Keating GM, Wellington K (2005) Bivalirudin: a review of its use in patients undergoing percutaneous coronary intervention. Drugs 65(13):1869–1891. https://doi.org/10.2165/00003495-200565130-00010
Moghadasi Z, Shahbazzadeh D, Jamili S et al (2020) Significant anticancer activity of a venom fraction derived from the persian gulf sea anemone, Stichodactyla Haddoni Iran. J Pharm Res 19(3):402–420. https://doi.org/10.22037/ijpr.2019.14600.12521
Moghaddam FD, Mortazavi P, Hamedi S et al (2020) Apoptotic effects of melittin on 4T1 breast cancer cell line is associated with up regulation of Mfn1 and Drp1 mRNA expression. Anticancer Agents Med Chem 20(7):790–799. https://doi.org/10.2174/1871520620666200211091451
Mohamed Abd El-Aziz T, Garcia Soares A, Stockand JD (2019) Snake venoms in drug discovery: valuable therapeutic tools for life saving. Toxins (Basel) 11:10. https://doi.org/10.3390/toxins11100564
Morel JL, Mokrzycki N, Lippens G et al (2022) Characterization of a family of scorpion toxins modulating Ca(2+)-activated Cl(-) current in vascular myocytes. Toxins (Basel) 14:11. https://doi.org/10.3390/toxins14110780
Morikawa N, Hagiwara K, Nakajima T (1992) Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem Biophys Res Commun 189(1):184–190. https://doi.org/10.1016/0006-291x(92)91542-x
Mouhat S, Visan V, Ananthakrishnan S et al (2005) K+ channel types targeted by synthetic OSK1, a toxin from Orthochirus scrobiculosus scorpion venom. Biochem J 385(Pt 1):95–104. https://doi.org/10.1042/BJ20041379
Mullooly M, McGowan PM, Crown J et al (2016) The ADAMs family of proteases as targets for the treatment of cancer. Cancer Biol Ther 17(8):870–880. https://doi.org/10.1080/15384047.2016.1177684
Munhoz J, Thome R, Rostami A et al (2021) The SNX-482 peptide from Hysterocrates gigas spider acts as an immunomodulatory molecule activating macrophages. Peptides 146:170648. https://doi.org/10.1016/j.peptides.2021.170648
Murugappan SK, Xie L, Wong HY et al (2021) Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats. Int J Mol Sci 22:15. https://doi.org/10.3390/ijms22157846
Natesh R, Schwager SL, Evans HR et al (2004) Structural details on the binding of antihypertensive drugs captopril and enalaprilat to human testicular angiotensin I-converting enzyme. Biochemistry 43(27):8718–8724. https://doi.org/10.1021/bi049480n
Ng XW, Huang Y, Liu KL et al (2014) In vitro evaluation of cenderitide-eluting stent I -an antirestenosis and proendothelization approach. J Pharm Sci 103(11):3631–3640. https://doi.org/10.1002/jps.24165
Nguyen CD, Lee G (2021) Neuroprotective activity of melittin-the main component of bee venom-against oxidative stress induced by abeta(25–35) in in vitro and in vivo models. Antioxidants (Basel) 10:11. https://doi.org/10.3390/antiox10111654
Nicolas S, Zoukimian C, Bosmans F et al (2019) Chemical synthesis, proper folding, Na(v) channel selectivity profile and analgesic properties of the spider peptide phlotoxin 1. Toxins (basel) 11:6. https://doi.org/10.3390/toxins11060367
Nishio H, Kumagaye KY, Kubo S et al (1993) Synthesis of omega-agatoxin IVA and its related peptides. Biochem Biophys Res Commun 196(3):1447–1453. https://doi.org/10.1006/bbrc.1993.2414
Notni J (2022) RGD Forever!—past, present, and future of a 3-letter-code in radiopharmacy and life sciences. Pharmaceuticals (Basel) 16:1. https://doi.org/10.3390/ph16010056
Nunes KP, Cordeiro MN, Richardson M et al (2010) Nitric oxide-induced vasorelaxation in response to PnTx2-6 toxin from Phoneutria nigriventer spider in rat cavernosal tissue. J Sex Med 7(12):3879–3888. https://doi.org/10.1111/j.1743-6109.2010.01978.x
O’Shea JC, Tcheng JE (2002) Eptifibatide: a potent inhibitor of the platelet receptor integrin glycoprotein IIb/IIIa. Expert Opin Pharmacother 3(8):1199–1210. https://doi.org/10.1517/14656566.3.8.1199
Ojeda PG, Wang CK, Craik DJ (2016) Chlorotoxin: Structure, activity, and potential uses in cancer therapy. Biopolymers 106(1):25–36. https://doi.org/10.1002/bip.22748
Olfa KZ, Jose L, Salma D et al (2005) Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis. Lab Invest 85(12):1507–1516. https://doi.org/10.1038/labinvest.3700350
Oliveira AL, Viegas MF, da Silva SL et al (2022) The chemistry of snake venom and its medicinal potential. Nat Rev Chem 6(7):451–469. https://doi.org/10.1038/s41570-022-00393-7
Oliveira CFB, Alves DP, Emerich BL et al (2019) Antinociceptive effect of PnTx4(5–5), a peptide from Phoneutria nigriventer spider venom, in rat models and the involvement of glutamatergic system. J Venom Anim Toxins Incl Trop Dis 25:e2019022. https://doi.org/10.1590/1678-9199-JVATITD-2019-0022
Oliveira CS, Torres MT, Pedron CN et al (2021) Synthetic peptide derived from scorpion venom displays minimal toxicity and anti-infective activity in an animal model. ACS Infect Dis 7(9):2736–2745. https://doi.org/10.1021/acsinfecdis.1c00261
Olivera BM, Miljanich GP, Ramachandran J et al (1994) Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem 63:823–867. https://doi.org/10.1146/annurev.bi.63.070194.004135
Painter NA, Morello CM, Singh RF et al (2013) An evidence-based and practical approach to using Bydureon in patients with type 2 diabetes. J Am Board Fam Med 26(2):203–210. https://doi.org/10.3122/jabfm.2013.02.120174
Paiva AL, Matavel A, Peigneur S et al (2016) Differential effects of the recombinant toxin PnTx4(5–5) from the spider Phoneutria nigriventer on mammalian and insect sodium channels. Biochimie 121:326–335. https://doi.org/10.1016/j.biochi.2015.12.019
Pan NC, Ma JJ, Peng HB (2012) Mechanosensitivity of nicotinic receptors. Pflugers Arch 464(2):193–203. https://doi.org/10.1007/s00424-012-1132-9
Pan NC, Zhang T, Hu S et al (2021) Fast desensitization of acetylcholine receptors induced by a spider toxin. Channels (austin) 15(1):507–515. https://doi.org/10.1080/19336950.2021.1961459
Park HG, Lee KS, Kim BY et al (2018) Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev Comp Immunol 85:51–60. https://doi.org/10.1016/j.dci.2018.04.001
Park JS, Kam TI, Lee S et al (2021) Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol Commun 9(1):78. https://doi.org/10.1186/s40478-021-01180-z
Parrish-Novak J, Byrnes-Blake K, Lalayeva N et al (2017) Nonclinical profile of BLZ-100, a tumor-targeting fluorescent imaging agent. Int J Toxicol 36(2):104–112. https://doi.org/10.1177/1091581817697685
Patocka J, Nepovimova E, Klimova B et al (2019) Antimicrobial peptides: amphibian host defense peptides. Curr Med Chem 26(32):5924–5946. https://doi.org/10.2174/0929867325666180713125314
Patridge E, Gareiss P, Kinch MS et al (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 21(2):204–207. https://doi.org/10.1016/j.drudis.2015.01.009
Peigneur S, da Costa Oliveira C, de Sousa Fonseca FC et al (2021) Small cyclic sodium channel inhibitors. Biochem Pharmacol 183:114291. https://doi.org/10.1016/j.bcp.2020.114291
Peng X, Zhou C, Hou X et al (2018) Molecular characterization and bioactivity evaluation of two novel bombinin peptides from the skin secretion of Oriental fire-bellied toad. Bombina Orientalis Amino Acids 50(2):241–253. https://doi.org/10.1007/s00726-017-2509-z
Pennington MW, Czerwinski A, Norton RS (2018) Peptide therapeutics from venom: current status and potential. Bioorg Med Chem 26(10):2738–2758. https://doi.org/10.1016/j.bmc.2017.09.029
Perumal Samy R, Stiles BG, Franco OL et al (2017) Animal venoms as antimicrobial agents. Biochem Pharmacol 134:127–138. https://doi.org/10.1016/j.bcp.2017.03.005
Piechowski-Jozwiak B, Abidi E, El Nekidy WS et al (2022) Clinical pharmacokinetics and pharmacodynamics of desmoteplase. Eur J Drug Metab Pharmacokinet 47(2):165–176. https://doi.org/10.1007/s13318-021-00743-8
Pinheiro-Junior EL, Boldrini-Franca J, de Campos Araujo LMP et al (2018) LmrBPP9: a synthetic bradykinin-potentiating peptide from Lachesis muta rhombeata venom that inhibits the angiotensin-converting enzyme activity in vitro and reduces the blood pressure of hypertensive rats. Peptides 102:1–7. https://doi.org/10.1016/j.peptides.2018.01.015
Pino A, Frattini F, Sun H et al (2021) Use of vivostat(R) autologous fibrin sealant in thyroid surgery. Surg Technol Int 3:57–61. https://doi.org/10.52198/21.STI.38.SO1441
Pompeia C, Frare EO, Peigneur S et al (2022) Synthetic polypeptide crotamine: characterization as a myotoxin and as a target of combinatorial peptides. J Mol Med (Berl) 100(1):65–76. https://doi.org/10.1007/s00109-021-02140-9
Pope JE, Deer TR (2013) Ziconotide: a clinical update and pharmacologic review. Expert Opin Pharmacother 14(7):957–966. https://doi.org/10.1517/14656566.2013.784269
Pucca MB, Bertolini TB, Cerni FA et al (2016a) Immunosuppressive evidence of Tityus serrulatus toxins Ts6 and Ts15: insights of a novel K(+) channel pattern in T cells. Immunology 147(2):240–250. https://doi.org/10.1111/imm.12559
Pucca MB, Cerni FA, Cordeiro FA et al (2016b) Ts8 scorpion toxin inhibits the Kv4.2 channel and produces nociception in vivo. Toxicon 119:244–252. https://doi.org/10.1016/j.toxicon.2016.06.014
Rajabnejad SH, Mokhtarzadeh A, Abnous K et al (2018) Targeted delivery of melittin to cancer cells by AS1411 anti-nucleolin aptamer. Drug Dev Ind Pharm 44(6):982–987. https://doi.org/10.1080/03639045.2018.1427760
Rasmussen S, Husted SE (2001) Tirofiban (Aggrastat). A non-peptide glycoprotein IIb/IIIa receptor inhibitor. Ugeskr Laeger. 163(4): 461–465.
Reimers C, Lee CH, Kalbacher H et al (2017) Identification of a cono-RFamide from the venom of Conus textile that targets ASIC3 and enhances muscle pain. Proc Natl Acad Sci USA 114(17):3507–3515. https://doi.org/10.1073/pnas.1616232114
Reis PVM, Boff D, Verly RM et al (2018) LyeTxI-b, a synthetic peptide derived from lycosa erythrognatha spider venom, shows potent antibiotic activity in vitro and in vivo. Front Microbiol 9:667. https://doi.org/10.3389/fmicb.2018.00667
Ren Y, Li C, Chang J et al (2018) Hi1a as a novel neuroprotective agent for ischemic stroke by inhibition of acid-sensing ion channel 1a. Transl Stroke Res 9(2):96–98. https://doi.org/10.1007/s12975-017-0575-x
Rezaei A, Asgari S, Komijani S et al (2022) Discovery of leptulipin, a new anticancer protein from theiranian scorpion, Hemiscorpius lepturus. Molecules 27:7. https://doi.org/10.3390/molecules27072056
Riciluca KCT, Oliveira UC, Mendonca RZ et al (2021) Rondonin: antimicrobial properties and mechanism of action. FEBS Open Biol 11(9):2541–2559. https://doi.org/10.1002/2211-5463.13253
Rigo FK, Dalmolin GD, Trevisan G et al (2013) Effect of omega-conotoxin MVIIA and Phalpha1beta on paclitaxel-induced acute and chronic pain. Pharmacol Biochem Behav 114–115:16–22. https://doi.org/10.1016/j.pbb.2013.10.014
Rincon-Cortes CA, Bayona-Rojas MA, Reyes-Montano EA et al (2022) Antimicrobial activity developed by scorpion venoms and its peptide component. Toxins (Basel) 14:11. https://doi.org/10.3390/toxins14110740
Ring J, Lonsdorf G, Schury W et al (1982) Bee and wasp venom allergy clinical aspects, prevention and therapy. MMW Munch Med Wochenschr 124(24):587–590
Roberts SS (2007) Symlin & Byetta. An injectable drug Q and A. Diabetes Forecast 60(7):23–25
Romero SM, Cardillo AB, Martinez Ceron MC et al (2020) Temporins: an approach of potential pharmaceutic candidates. Surg Infect (larchmt) 21(4):309–322. https://doi.org/10.1089/sur.2019.266
Rong M, Chen J, Tao H et al (2011) Molecular basis of the tarantula toxin jingzhaotoxin-III (beta-TRTX-Cj1alpha) interacting with voltage sensors in sodium channel subtype Nav1.5. FASEB J 25(9):3177–3185. https://doi.org/10.1096/fj.10-178848
Salimi A, Rahimitabar N, Vazirizadeh A et al (2021) Persian Gulf Snail Crude Venom (Conus textile): a potential source of anti-cancer therapeutic agents for glioblastoma through mitochondrial-mediated apoptosis. Asian Pac J Cancer Prev 22(S1):49–57. https://doi.org/10.31557/APJCP.2021.22.S1.49
Savelyeva A, Ghavami S, Davoodpour P et al (2014) An overview of Brevinin superfamily: structure, function and clinical perspectives. Adv Exp Med Biol 818:197–212. https://doi.org/10.1007/978-1-4471-6458-6_10
Scharfenberg F, Helbig A, Sammel M et al (2020) Degradome of soluble ADAM10 and ADAM17 metalloproteases. Cell Mol Life Sci 77(2):331–350. https://doi.org/10.1007/s00018-019-03184-4
Scheen AJ (2014) Bydureon: first once weekly GLP-1 receptor agonist (exenatide LAR). Rev Med Liege 69(4):214–219
Scholle JM, White CM (2010) Bivalirudin: a review of pharmacology and therapeutic use. Conn Med 74(10):595–597
Schonthal AH, Swenson SD, Chen TC et al (2020) Preclinical studies of a novel snake venom-derived recombinant disintegrin with antitumor activity: a review. Biochem Pharmacol 181:114149. https://doi.org/10.1016/j.bcp.2020.114149
Schwartsmann G, DiLeone LP, Horowitz M et al (2006) A phase I trial of the bombesin/gastrin-releasing peptide (BN/GRP) antagonist RC3095 in patients with advanced solid malignancies. Invest New Drugs 24(5):403–412. https://doi.org/10.1007/s10637-006-6886-5
Schwartsmann G, Ratain MJ, Cragg GM et al (2002) Anticancer drug discovery and development throughout the world. J Clin Oncol 20(18):47S-59S
Sharma S, Bhambi B, Nyitray W et al (2003) Bivalirudin (Angiomax) use during intracoronary brachytherapy may predispose to acute closure. J Cardiovasc Pharmacol Ther 8(1):9–15. https://doi.org/10.1177/107424840300800i103
Shi P, Xie S, Yang J et al (2022) Pharmacological effects and mechanisms of bee venom and its main components: recent progress and perspective. Front Pharmacol. 13:1001553. https://doi.org/10.3389/fphar.2022.1001553
Shrestha A, Lahooti B, Mikelis CM et al (2022) Chlorotoxin and lung cancer: a targeting perspective for drug delivery. Pharmaceutics 14:12. https://doi.org/10.3390/pharmaceutics14122613
Si H, Yin C, Wang W et al (2022) Effect of the snake venom component crotamine on lymphatic endothelial cell responses and lymph transport. Microcirculation. https://doi.org/10.1111/micc.12775
Siigur J, Aaspollu A, Siigur E (2019) Biochemistry and pharmacology of proteins and peptides purified from the venoms of the snakes Macrovipera lebetina subspecies. Toxicon 158:16–32. https://doi.org/10.1016/j.toxicon.2018.11.294
Siigur J, Siigur E (2022) Biochemistry and toxicology of proteins and peptides purified from the venom of Vipera berus berus. Toxicon X. 15: 131. https://doi.org/10.1016/j.toxcx.2022.100131
Silva CND, Lomeo RS, Torres FS et al (2018) PnTx2-6 (or delta-CNTX-Pn2a), a toxin from Phoneutria nigriventer spider venom, releases l-glutamate from rat brain synaptosomes involving Na(+) and Ca(2+) channels and changes protein expression at the blood-brain barrier. Toxicon 150:280–288. https://doi.org/10.1016/j.toxicon.2018.06.073
Silva CND, Silva FRD, Dourado LFN et al (2019) A New topical eye drop containing LyeTxI-b, a synthetic peptide designed from A lycosa erithrognata venom toxin, was effective to treat resistant bacterial keratitis. Toxins (Basel) 11:4. https://doi.org/10.3390/toxins11040203
Silva FR, Batista EM, Gomez MV et al (2016) The Phoneutria nigriventer spider toxin, PnTx4-5-5, promotes neuronal survival by blocking NMDA receptors. Toxicon 112:16–21. https://doi.org/10.1016/j.toxicon.2016.01.056
Silva, M.; Vinck, R.; Wang, Y. et al (2023). Towards Selective Delivery of a Ruthenium(II) Polypyridyl Complex-Containing Bombesin Conjugate into Cancer Cells. Chembiochem. 24(4): e202200647. doi:: https://doi.org/10.1002/cbic.202200647
Silva MFP, Alves PL, Alponti RF et al (2019) Effects of obesity induced by high-calorie diet and its treatment with exenatide on muscarinic acetylcholine receptors in rat hippocampus. Biochem Pharmacol 169:113630. https://doi.org/10.1016/j.bcp.2019.113630
Simmaco M, Kreil G, Barra D (2009) Bombinins, antimicrobial peptides from Bombina species. Biochim Biophys Acta 1788(8):1551–1555. https://doi.org/10.1016/j.bbamem.2009.01.004
Simoes-Silva R, Alfonso J, Gomez A et al (2018) Snake venom, a natural library of new potential therapeutic molecules: challenges and current perspectives. Curr Pharm Biotechnol 19(4):308–335. https://doi.org/10.2174/1389201019666180620111025
Sitges M, Galindo CA (2005) Omega-agatoxin-TK is a useful tool to study P-type Ca2+ channel-mediated changes in internal Ca2+ and glutamate release in depolarised brain nerve terminals. Neurochem Int 46(1):53–60. https://doi.org/10.1016/j.neuint.2004.07.004
Soares-Silva B, Beserra-Filho JIA, Morera PMA et al (2022) The bee venom active compound melittin protects against bicuculline-induced seizures and hippocampal astrocyte activation in rats. Neuropeptides 91:102209. https://doi.org/10.1016/j.npep.2021.102209
Soltan-Alinejad P, Alipour H, Meharabani D et al (2022) Therapeutic potential of bee and scorpion venom phospholipase A2 (PLA2): a narrative review. Iran J Med Sci 47(4):300–313. https://doi.org/10.30476/IJMS.2021.88511.1927
Soltaninejad H, Zare-Zardini H, Ordooei M et al (2021) Antimicrobial peptides from amphibian innate immune system as potent antidiabetic agents: a literature review and bioinformatics analysis. J Diabetes Res 2021:2894722. https://doi.org/10.1155/2021/2894722
Song Y, Lu Y, Wang L et al (2009) Purification, characterization and cloning of two novel tigerinin-like peptides from skin secretions of Fejervarya cancrivora. Peptides 30(7):1228–1232. https://doi.org/10.1016/j.peptides.2009.03.020
Sonnevend A, Knoop FC, Patel M et al (2004) Antimicrobial properties of the frog skin peptide, ranatuerin-1 and its [Lys-8]-substituted analog. Peptides 25(1):29–36. https://doi.org/10.1016/j.peptides.2003.11.011
Sorrieul J, Robert J, Dupoiron D et al (2021) Stability study of admixtures combining ziconotide with morphine or sufentanil in polypropylene syringes. Neuromodulation 24(7):1145–1156. https://doi.org/10.1111/ner.13289
Sousa SR, Wingerd JS, Brust A et al (2017) Discovery and mode of action of a novel analgesic beta-toxin from the African spider Ceratogyrus darlingi. PLoS ONE 12(9):e01848. https://doi.org/10.1371/journal.pone.0182848
Spira ME, Hasson A, Fainzilber M et al (1993) Chemical and electrophysiological characterization of new peptide neurotoxins from the venom of the molluscivorous snail Conus textile neovicarius: a review. Isr J Med Sci 29(9):530–543
Sri Balasubashini M, Karthigayan S, Somasundaram ST et al (2006a) FV peptide induces apoptosis in HEp 2 and HeLa cells: an insight into the mechanism of induction. J Carcinog 5:27. https://doi.org/10.1186/1477-3163-5-27
Sri Balasubashini M, Karthigayan S, Somasundaram ST et al (2006b) Fish venom (Pterios volitans) peptide reduces tumor burden and ameliorates oxidative stress in Ehrlich’s ascites carcinoma xenografted mice. Bioorg Med Chem Lett 16(24):6219–6225. https://doi.org/10.1016/j.bmcl.2006.09.025
Srinivasan D, Ojo OO, Abdel-Wahab YH et al (2014) Insulin-releasing and cytotoxic properties of the frog skin peptide, tigerinin-1R: a structure-activity study. Peptides 55:23–31. https://doi.org/10.1016/j.peptides.2014.02.002
Stewart JM (2020) TRPV6 as a target for cancer therapy. J Cancer 11(2):374–387. https://doi.org/10.7150/jca.31640
Stocker K, Barlow GH (1976) The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol 45:214–223. https://doi.org/10.1016/s0076-6879(76)45021-8
Straub A, Azevedo R, Beierlein W et al (2006) Tirofiban (Aggrastat) protects platelets and decreases platelet-granulocyte binding in an extracorporeal circulation model. Thorac Cardiovasc Surg 54(3):162–167. https://doi.org/10.1055/s-2005-872952
Straub PW, Harder A (1971) Behavior of I I 125 labeled fibrinogen during therapeutic defibrination with highly purified reptilase (“Defibrase”). Schweiz Med Wochenschr 101(49):1802–1804
Sun M, Han X, Liu X et al (2023) Cinobufacini suppresses malignant behaviors of endometrial cancer by regulating NF-kappaB pathway. Biotechnol Genet Eng Rev. https://doi.org/10.1080/02648725.2023.2199236
Swaim MW, Chiang HS, Huang TF (1996) Characterisation of platelet aggregation induced by PC-3 human prostate adenocarcinoma cells and inhibited by venom peptides, trigramin and rhodostomin. Eur J Cancer 32A(4):715–721. https://doi.org/10.1016/0959-8049(95)00648-6
Swiatly-Blaszkiewicz A, Mrowczynska L, Matuszewska E et al (2020) The effect of bee venom peptides melittin, tertiapin, and apamin on the human erythrocytes ghosts: a preliminary study. Metabolites 10:5. https://doi.org/10.3390/metabo10050191
Tajti G, Wai DCC, Panyi G et al (2020) The voltage-gated potassium channel K(V)1.3 as a therapeutic target for venom-derived peptides. Biochem Pharmacol 18:114146. https://doi.org/10.1016/j.bcp.2020.114146
Tan X, Liang X, Xi J et al (2021) Clinical efficacy and safety of Huachansu injection combination with platinum-based chemotherapy for advanced non-small cell lung cancer: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 100(36):e27161. https://doi.org/10.1097/MD.0000000000027161
Tanabe S, Fu J, Cang J (2022) Strong tuning for stereoscopic depth indicates orientation-specific recurrent circuitry in tree shrew V1. Curr Biol. https://doi.org/10.1016/j.cub.2022.10.063
Tang F, Wu H, Zheng J et al (2021) Effect of melittin on iNOS and NF-kappaB expression induced by IL-1betain C518 cells. Pak J Pharm Sci 34(1):95–101
Tang S, Zhou L, He H et al (2022) MnO(2)-melittin nanoparticles serve as an effective anti-tumor immunotherapy by enhancing systemic immune response. Biomaterials 288:121706. https://doi.org/10.1016/j.biomaterials.2022.121706
Tangthong T, Piroonpan T, Thipe VC et al (2021) Water-soluble chitosan conjugated DOTA-bombesin peptide capped gold nanoparticles as a targeted therapeutic agent for prostate cancer. Nanotechnol Sci Appl 14:69–89. https://doi.org/10.2147/NSA.S301942
Tanner MR, Tajhya RB, Huq R et al (2017) Prolonged immunomodulation in inflammatory arthritis using the selective Kv1.3 channel blocker HsTX1[R14A] and its PEGylated analog. Clin Immunol 180:45–57. https://doi.org/10.1016/j.clim.2017.03.014
Tarcha EJ, Chi V, Munoz-Elias EJ et al (2012) Durable pharmacological responses from the peptide ShK-186, a specific Kv1.3 channel inhibitor that suppresses T cell mediators of autoimmune disease. J Pharmacol Exp Ther 342(3):642–653. https://doi.org/10.1124/jpet.112.191890
Tarcha EJ, Olsen CM, Probst P et al (2017) Safety and pharmacodynamics of dalazatide, a Kv13 channel inhibitor, in the treatment of plaque psoriasis: a randomized phase 1b trial. PLoS ONE 12(7):e0180762. https://doi.org/10.1371/journal.pone.0180762
Tawfik, M.M.; Bertelsen, M.; Abdel-Rahman, M.A. et al (2021). Scorpion Venom Antimicrobial Peptides Induce Siderophore Biosynthesis and Oxidative Stress Responses in Escherichia coli. mSphere. 6(3. doi:: https://doi.org/10.1128/mSphere.00267-21
Tender T, Rahangdale RR, Balireddy S et al (2021) Melittin, a honeybee venom derived peptide for the treatment of chemotherapy-induced peripheral neuropathy. Med Oncol 38(5):52. https://doi.org/10.1007/s12032-021-01496-9
Teodoro A, Goncalves FJM, Oliveira H et al (2022) Venom of viperidae: a perspective of its antibacterial and antitumor potential. Curr Drug Targets 23(2):126–144. https://doi.org/10.2174/1389450122666210811164517
Thelwell C, Rigsby P, Locke M et al (2018) An international collaborative study to calibrate the WHO 2nd International Standard for Ancrod (15/106) and the WHO Reference Reagent for Batroxobin (15/140): communication from the SSC of the ISTH. J Thromb Haemost 16(5):1003–1006. https://doi.org/10.1111/jth.13996
Tian J, Paquette-Straub C, Sage EH et al (2007) Inhibition of melanoma cell motility by the snake venom disintegrin eristostatin. Toxicon 49(7):899–908. https://doi.org/10.1016/j.toxicon.2006.12.013
Tome Y, Kimura H, Kiyuna T et al (2016a) Disintegrin targeting of an alphavbeta3 integrin-over-expressing high-metastatic human osteosarcoma with echistatin inhibits cell proliferation, migration, invasion and adhesion in vitro. Oncotarget 7(29):46315–46320. https://doi.org/10.18632/oncotarget.10111
Tome Y, Kimura H, Sugimoto N et al (2016b) The disintegrin echistatin in combination with doxorubicin targets high-metastatic human osteosarcoma overexpressing alphanubeta3 integrin in chick embryo and nude mouse models. Oncotarget 7(52):87031–87036. https://doi.org/10.18632/oncotarget.13497
Tornesello AL, Buonaguro L, Tornesello ML et al (2017a) New insights in the design of bioactive peptides and chelating agents for imaging and therapy in oncology. Molecules 22:8. https://doi.org/10.3390/molecules22081282
Tornesello AL, Tornesello ML, Buonaguro FM (2017b) An overview of bioactive peptides for in vivo imaging and therapy in human diseases. Mini Rev Med Chem 17(9):758–770. https://doi.org/10.2174/1389557517666170120151739
Tornesello ML, Buonaguro L, Buonaguro FM (2015) An overview of new biomolecular pathways in pathogen-related cancers. Future Oncol 11(11):1625–1639. https://doi.org/10.2217/fon.15.87
Tran HNT, McMahon KL, Deuis JR et al (2022) Structural and functional insights into the inhibition of human voltage-gated sodium channels by mu-conotoxin KIIIA disulfide isomers. J Biol Chem 298(3):101728. https://doi.org/10.1016/j.jbc.2022.101728
Trevisan G, Oliveira SM (2022) Animal venom peptides cause antinociceptive effects by voltage-gated calcium channels activity blockage. Curr Neuropharmacol 20(8):1579–1599. https://doi.org/10.2174/1570159X19666210713121217
Trim CM, Byrne LJ, Trim SA (2021) Utilisation of compounds from venoms in drug discovery. Prog Med Chem 60:1–66. https://doi.org/10.1016/bs.pmch.2021.01.001
Umana IC, Daniele CA, McGehee DS (2013) Neuronal nicotinic receptors as analgesic targets: it’s a winding road. Biochem Pharmacol 86(8):1208–1214. https://doi.org/10.1016/j.bcp.2013.08.001
Utkin Y (2021) Animal venoms and their components: molecular mechanisms of action. Toxins (Basel) 13:6. https://doi.org/10.3390/toxins13060415
Utkin Y, Vassilevski A, Kudryavtsev D et al (2019) Editorial: animal toxins as comprehensive pharmacological tools to identify diverse ion channels. Front Pharmacol 10:423. https://doi.org/10.3389/fphar.2019.00423
Utkin YN (2017) Modern trends in animal venom research - omics and nanomaterials. World J Biol Chem 8(1):4–12. https://doi.org/10.4331/wjbc.v8.i1.4
Utkin YN (2019) Aging affects nicotinic acetylcholine receptors in brain. Cent Nerv Syst Agents Med Chem 19(2):119–124. https://doi.org/10.2174/1871524919666190320102834
Uzair B, Atlas N, Malik SB et al (2018) Snake venom as an effective tool against colorectal cancer. Protein Pept Lett 25(7):626–632. https://doi.org/10.2174/0929866525666180614112935
Valverde P, Kawai T, Taubman MA (2004) Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J Bone Miner Res 19(1):155–164. https://doi.org/10.1359/JBMR.0301213
Van De Car DA, Rao SV, Ohman EM (2010) Bivalirudin: a review of the pharmacology and clinical application. Expert Rev Cardiovasc Ther 8(12):1673–1681. https://doi.org/10.1586/erc.10.158
Vannini E, Mori E, Tantillo E et al (2021) CTX-CNF1 recombinant protein selectively targets glioma cells in vivo. Toxins (Basel) 13:3. https://doi.org/10.3390/toxins13030194
Varga JFA, Bui-Marinos MP, Katzenback BA (2018) Frog skin innate immune defences: sensing and surviving pathogens. Front Immunol 9:3128. https://doi.org/10.3389/fimmu.2018.03128
Vasconcelos AA, Estrada JC, David V et al (2021) Structure-function relationship of the disintegrin family: sequence signature and integrin interaction. Front Mol Biosci 8:783301. https://doi.org/10.3389/fmolb.2021.783301
Veytia-Bucheli JI, Jimenez-Vargas JM, Melchy-Perez EI et al (2018) K(v)1.3 channel blockade with the Vm24 scorpion toxin attenuates the CD4(+) effector memory T cell response to TCR stimulation. Cell Commun Signal 16(1):45. https://doi.org/10.1186/s12964-018-0257-7
Voelter K, Tappeiner C, Klein K et al (2019) Fibrinolytic capacity of desmoteplase compared to tissue plasminogen activator in rabbit eyes. J Ocul Pharmacol Ther 35(1):66–75. https://doi.org/10.1089/jop.2018.0070
Wade D, Silberring J, Soliymani R et al (2000) Antibacterial activities of temporin A analogs. FEBS Lett 479(1–2):6–9. https://doi.org/10.1016/s0014-5793(00)01754-3
Waldron NH, Dallas T, Erhunmwunsee L et al (2017) Bleeding risk associated with eptifibatide (Integrilin) bridging in thoracic surgery patients. J Thromb Thrombolysis 43(2):194–202. https://doi.org/10.1007/s11239-016-1441-5
Wang A, Zheng Y, Zhu W et al (2022) Melittin-based nano-delivery systems for cancer therapy. Biomolecules. https://doi.org/10.3390/biom12010118
Wang C, Geng Z, Li P et al (2019a) Prokaryotic expression and hypoglycemic activity determination of insulin G1 from Conus geographus. Sheng Wu Gong Cheng Xue Bao 35(3):505–512. https://doi.org/10.13345/j.cjb.180280
Wang D, Starr R, Chang WC et al (2020a) Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med 12:533. https://doi.org/10.1126/scitranslmed.aaw2672
Wang J, Irnaten M, Mendelowitz D (2001) Agatoxin-IVA-sensitive calcium channels mediate the presynaptic and postsynaptic nicotinic activation of cardiac vagal neurons. J Neurophysiol 85(1):164–168. https://doi.org/10.1152/jn.2001.85.1.164
Wang M, Wang Y, Wang A et al (2010) Five novel antimicrobial peptides from skin secretions of the frog. Amolops Loloensis Comput Biochem Physiol B 155(1):72–76. https://doi.org/10.1016/j.cbpb.2009.10.003
Wang T, Jiang Y, Chen X et al (2020b) Ranacyclin-NF, a Novel Bowman-Birk type protease inhibitor from the skin secretion of the East Asian frog, Pelophylax nigromaculatus. Biology (Basel). https://doi.org/10.3390/biology9070149
Wang Y, Li K, Han S et al (2019b) Chlorotoxin targets ERalpha/VASP signaling pathway to combat breast cancer. Cancer Med 8(4):1679–1693. https://doi.org/10.1002/cam4.2019
Warkentin TE, Koster A (2005) Bivalirudin: a review. Expert Opin Pharmacother 6(8):1349–1371. https://doi.org/10.1517/14656566.6.8.1349
Warrell DA (2017) Snakebites: reducing their international impact. Med J Aust 207(3):112–113. https://doi.org/10.5694/mja17.00480
Warrell DA (2019) Venomous bites, stings, and poisoning: an update. Infect Dis Clin North Am 33(1):17–38. https://doi.org/10.1016/j.idc.2018.10.001
Weber KC, Honório KM, Da Silva SL et al (2005) Selection of quantum chemical descriptors by chemometric methods in the study of antioxidant activity of flavonoid compounds. Int J Quantum Chem 103(5):731–737
Wei H, Xie Z, Tan X et al (2020) Temporin-like peptides show antimicrobial and anti-biofilm activities against streptococcus mutans with reduced hemolysis. Molecules 25:23. https://doi.org/10.3390/molecules25235724
White HD, Ellis CJ, French JK et al (1998) Hirudin (desirudin) and Hirulog (bivalirudin) in acute ischaemic syndromes and the rationale for the Hirulog/Early Reperfusion Occlusion (HERO-2) Study. Aust N Z J Med 28(4):551–554. https://doi.org/10.1111/j.1445-5994.1998.tb02109.x
Whyte MB, Shojaee-Moradie F, Sharaf SE et al (2019) Lixisenatide reduces chylomicron triacylglycerol by increased clearance. J Clin Endocrinol Metab 104(2):359–368. https://doi.org/10.1210/jc.2018-01176
Wie, C.S. and Derian, A. Ziconotide. StatPearls. Treasure Island (FL)2022.
Wie, C.S. and Derian, A. Ziconotide. StatPearls. Treasure Island (FL)2023.
Wiere S, Sugai C, Espiritu MJ et al (2022) Research into the bioengineering of a novel alpha-conotoxin from the milked venom of Conus obscurus. Int J Mol Sci 23:20. https://doi.org/10.3390/ijms232012096
Wojta J (2016) Cenderitide: a multivalent designer-peptide-agonist of particulate guanylyl cyclase receptors with considerable therapeutic potential in cardiorenal disease states. Eur Heart J Cardiovasc Pharmacother 2(2):106–107. https://doi.org/10.1093/ehjcvp/pvv043
Wu H, Cheng H, Luo S et al (2022) Use of cellular metabolomics and lipidomics to decipher the mechanism of Huachansu injection-based intervention against human hepatocellular carcinoma cells. J Pharm Biomed Anal. 212:14654. https://doi.org/10.1016/j.jpba.2022.114654
Wu Q, Wang SP, Sun XX et al (2022) HuaChanSu suppresses tumor growth and interferes with glucose metabolism in hepatocellular carcinoma cells by restraining Hexokinase-2. Int J Biochem Cell Biol 142:106123. https://doi.org/10.1016/j.biocel.2021.106123
Xiang J, Zhou M, Wu Y et al (2017) The synergistic antimicrobial effects of novel bombinin and bombinin H peptides from the skin secretion of Bombina orientalis. Biosci Rep 37:5
Xiao G, Liu J, Peng L et al (2022) Compositional and toxicological investigation of pooled venom from farm-raised Naja atra. J Venom Anim Toxins Incl Trop Dis. 28:e20210040. https://doi.org/10.1590/1678-9199-JVATITD-2021-0040
Xie C, Fan Y, Yin S et al (2021) Novel amphibian-derived antioxidant peptide protects skin against ultraviolet irradiation damage. J Photochem Photobiol B 224:112327. https://doi.org/10.1016/j.jphotobiol.2021.112327
Xie X, Li Y, Zhu H et al (2022) Melittin inhibits growth of human osteosarcoma 143B cells through induction of apoptosis via suppressing the Wnt/beta-catenin signaling pathway. Anticancer Agents Med Chem 22(18):3172–3181. https://doi.org/10.2174/1871520622666220509121627
Xiong S, Huang C (2018) Synergistic strategies of predominant toxins in snake venoms. Toxicol Lett 287:142–154. https://doi.org/10.1016/j.toxlet.2018.02.004
Yaacoub C, Wehbe R, Salma Y et al (2022) Apis mellifera syriaca venom: evaluation of its anticoagulant effect, proteolytic activity, and cytotoxicity along with its two main compounds-MEL and PLA2-on hela cancer cells. Molecules 27:5. https://doi.org/10.3390/molecules27051653
Yamada M, Miller DM, Lowe M et al (2021) A first-in-human study of BLZ-100 (tozuleristide) demonstrates tolerability and safety in skin cancer patients. Contemp Clin Trials Commun 23:100830. https://doi.org/10.1016/j.conctc.2021.100830
Yang A, Wu Q, Chen Q et al (2022) Cinobufagin restrains the growth and triggers DNA damage of human hepatocellular carcinoma cells via proteasome-dependent degradation of thymidylate synthase. Chem Biol Interact 360:109938. https://doi.org/10.1016/j.cbi.2022.109938
Yang S, Xiao Y, Kang D et al (2013) Discovery of a selective NaV1.7 inhibitor from centipede venom with analgesic efficacy exceeding morphine in rodent pain models. Proc Natl Acad Sci USA 110(43):17534–17539. https://doi.org/10.1073/pnas.1306285110
Yang X, Feng P, Ji R et al (2022b) Therapeutic application of GLP-1 and GIP receptor agonists in Parkinson’s disease. Expert Opin Ther Targets 26(5):445–460. https://doi.org/10.1080/14728222.2022.2079492
Yantis MA, O'Toole KN, Ring P (2009) Leech therapy. Am J Nurs 109(4):36–42; quiz 43. https://doi.org/10.1097/01.NAJ.0000348601.01489.77
Ye X, Guan S, Liu J et al (2016) Activities of venom proteins and peptides with possible therapeutic applications from bees and WASPS. Protein Pept Lett 23(8):748–755. https://doi.org/10.2174/0929866523666160618120824
Yin JB, Liu HX, Dong QQ et al (2023) Correlative increasing expressions of KIF5b and Nav1.7 in DRG neurons of rats under neuropathic pain conditions. Physiol Behav. 263:4115. https://doi.org/10.1016/j.physbeh.2023.114115
Yin S, Wang Y, Liu N et al (2019) Potential skin protective effects after UVB irradiation afforded by an antioxidant peptide from Odorrana andersonii. Biomed Pharmacother 120:109535. https://doi.org/10.1016/j.biopha.2019.109535
Yu M, Chen Q, Li Q et al (2022) A disintegrin and metalloprotease 10 expressions modulate potential metastatic and thrombus formation in pancreatic carcinoma. Iran J Public Health 51(8):1778–1789. https://doi.org/10.18502/ijph.v51i8.10262
Zakian A, Ahmadi HA, Keleshteri MH et al (2022) Study on the effect of medicinal leech therapy (Hirudo medicinalis) on full-thickness excisional wound healing in the animal model. Res Vet Sci 153:153–168. https://doi.org/10.1016/j.rvsc.2022.10.015
Zambelli VO, Fernandes AC, Gutierrez VP et al (2014) Peripheral sensitization increases opioid receptor expression and activation by crotalphine in rats. PLoS ONE 9(3):e90576. https://doi.org/10.1371/journal.pone.0090576
Zeng C, Wu L, Zhao Y et al (2018) Tea saponin reduces the damage of Ectropis obliqua to tea crops, and exerts reduced effects on the spiders Ebrechtella tricuspidata and Evarcha albaria compared to chemical insecticides. PeerJ 6:4534. https://doi.org/10.7717/peerj.4534
Zeng L, Hou J, Ge C et al (2022) Clinical study of anti-snake venom blockade in the treatment of local tissue necrosis caused by Chinese cobra (Naja atra) bites. PLoS Negl Trop Dis 16(12):e0010997. https://doi.org/10.1371/journal.pntd.0010997
Zhang F, Zhang C, Xu X et al (2019) Naja atra venom peptide reduces pain by selectively blocking the voltage-gated sodium channel Nav1.8. J Biol Chem 294(18):7324–7334. https://doi.org/10.1074/jbc.RA118.007370
Zhang HL, Han R, Chen ZX et al (2006) Analgesic effects of receptin, a chemically modified cobratoxin from Thailand cobra venom. Neurosci Bull 22(5):267–273
Zhang J, Shi Y, Huang Z et al (2022) Structural basis for Na(V)1.7 inhibition by pore blockers. Nat Struct Mol Biol 29(12):1208–1216. https://doi.org/10.1038/s41594-022-00860-1
Zhang W, Zhang J, Cheng L et al (2018a) A disintegrin and metalloprotease 10-containing exosomes derived from nasal polyps promote angiogenesis and vascular permeability. Mol Med Rep 17(4):5921–5927. https://doi.org/10.3892/mmr.2018.8634
Zhang YS, Weng WY, Xie BC et al (2018b) Glucagon-like peptide-1 receptor agonists and fracture risk: a network meta-analysis of randomized clinical trials. Osteoporos Int 29(12):2639–2644. https://doi.org/10.1007/s00198-018-4649-8
Zhang YX, Peng DZ, Zhang QF et al (2019b) micro-TRTX-Ca1a: a novel neurotoxin from Cyriopagopus albostriatus with analgesic effects. Acta Pharmacol Sin 40(7):859–866. https://doi.org/10.1038/s41401-018-0181-9
Zhang Z, Mu L, Tang J et al (2013) A small peptide with therapeutic potential for inflammatory acne vulgaris. PLoS ONE 8(8):e72923. https://doi.org/10.1371/journal.pone.0072923
Zhao L, Zhu J, Gong J et al (2020) Polyethylenimine-based theranostic nanoplatform for glioma-targeting single-photon emission computed tomography imaging and anticancer drug delivery. J Nanobiotechnol 18(1):143. https://doi.org/10.1186/s12951-020-00705-3
Zhong Y, Song B, Mo G et al (2014) A novel neurotoxin from venom of the spider, Brachypelma albopilosum. PLoS ONE 9(10):e110221. https://doi.org/10.1371/journal.pone.0110221
Zhou J, Wang A, Cai T et al (2022) Integrin alpha3/alpha6 and alphaV are implicated in ADAM15-activated FAK and EGFR signalling pathway individually and promote non-small-cell lung cancer progression. Cell Death Dis 13(5):486. https://doi.org/10.1038/s41419-022-04928-0
Zhou X, Liu Y, Gao Y et al (2020) Enhanced antimicrobial activity of N-terminal derivatives of a novel brevinin-1 peptide from the skin secretion of Odorrana schmackeri. Toxins (Basel) 12:8. https://doi.org/10.3390/toxins12080484
Zhou X, Shi D, Zhong R et al (2019) Bioevaluation of ranatuerin-2Pb from the frog skin secretion of Rana pipiens and its truncated analogues. Biomolecules 9:6. https://doi.org/10.3390/biom9060249
Zhou XD, Ding CH, Tai H et al (2004) A novel disintegrin, jerdonatin, inhibits platelet aggregation and sperm-egg binding. Comput Biochem Physiol B 139(1):117–122. https://doi.org/10.1016/j.cbpc.2004.06.012
Zhu J, Lin F-Y, Zhu J (2022a). Integrin alaphIIBbeta3 complex with Eptifibatide. https://doi.org/10.2210/pdb7THO/pdb
Zhu J, Lin F-Y, Zhu J et al (2022b) Integrin alaphIIBbeta3 complex with Tirofiban. https://doi.org/10.2210/pdb7td8/pdb
Zhu S, Gao B, Peigneur S et al (2020) How a scorpion toxin selectively captures a prey sodium channel: the molecular and evolutionary basis uncovered. Mol Biol Evol 37(11):3149–3164. https://doi.org/10.1093/molbev/msaa152
Acknowledgements
We acknowledge the financial support. (1) FCT - Foundation for Science and Technology of Portugual; 2) Coordination for the Improvement of Higher Education Personnel (CAPES), National Council for Scientific and Technological Development (CNPq); 3) Foundation for Support to the Development of Scientific and Technological Actions and Research of Rondônia (FAPERO); 4) PDTIS/FIOCRUZ platforms and Laboratory of Biotechnology of Proteins and Bioactive Compounds (LABIOPROT), Oswaldo Cruz Foundation (FIOCRUZ), Porto Velho, RO, Brazil. 5) CalPhotos University of California, Berkeley. (6) P. Gowri Shankar from Kalinga Foundation, India.
Funding
Open access funding provided by FCT|FCCN (b-on). Indicated in the item "Acknowledgments".
Author information
Authors and Affiliations
Contributions
All authors had the same responsibilities and contributions in this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate.
Not applicable.
Consent for publication
All authors consent the publication of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Oliveira, A.N., Soares, A.M. & Da Silva, S.L. Why to Study Peptides from Venomous and Poisonous Animals?. Int J Pept Res Ther 29, 76 (2023). https://doi.org/10.1007/s10989-023-10543-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10989-023-10543-0