Abstract
Context
Forests are increasingly fragmented, and as a result most forests in the United States are within one km of an edge. Edges change environmental conditions of the forest—especially radiation, roughness, temperature, and moisture—that can have consequences for plant productivity and ecosystem functions. However, edge effects on aboveground characteristics of plants and the environment are better understood relative to plant roots and soil in the belowground environment.
Objectives
Our main objectives were to determine if soil C pools and fluxes are higher at the edge relative to other landscape positions, and to understand how specific belowground processes contribute to bulk differences in pools and fluxes.
Methods
We measured environmental conditions, live and dead fine root traits, soil chemistry, and soil respiration along a 75 m transect from interior forest to meadow in Gaithersburg, MD.
Results
We observed differences in the soil chemical, biological and hydrological environment between the forest interior, edge and adjacent meadow. In some cases, the forest edge represented a mid-point in environmental or belowground characteristics between the forest interior and meadow (e.g., pH, C-to-N ratio [C:N], live fine root biomass, heterotrophic respiration), likely reflecting the change in litter type and quality associated with the transition from grass to woody species. In other cases, neighboring landscape positions were different from the forest edge, which was drier and had higher dead fine root biomass. Although soil C contents were not significantly different across landscape positions, there was a tendency towards higher average soil C content at the edge relative to other landscape positions, suggesting that increased C loss related to root decay and greater soil respiration at the edge relative to the forest interior may have been offset by increased C gain from high plant productivity and subsequent inputs to soil.
Conclusions
This research provides insight into how forest edge environments may differ from the interior and how concurrent processes above- and belowground may contribute to those differences.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Globally, forest fragmentation is ubiquitous with most forested land found within one km of an edge (Haddad et al. 2015). In the eastern United States, since European colonization in the 1600s forested land has undergone many transitions, including extensive logging, clearing for agriculture, abandonment, and urban development (Thompson et al. 2013). When forests are fragmented, the smaller and disjointed patch sizes commonly result in loss of biodiversity (Zuidema et al. 1996), although the forest edge in particular can have greater species diversity and non-native abundance (Franklin et al. 2021). Because the forest edge abuts a non-forest environment, the physical condition of a forest edge ecotone can differ greatly from the forest interior, with consequences for the overall carbon (C), biogeochemical, and hydrological cycles of the landscape (Smith et al. 2018).
Forest edges typically experience increased solar radiation, which is often accompanied by changes in surface roughness, temperature and soil moisture (Smith et al. 2018). There have been many studies documenting changes to aboveground plant productivity that differ by biome. In temperate forests such as those in the northeastern US, creation of forest edges is observed to increase basal area increment (Morreale et al. 2021; Reinmann and Hutyra 2017; Briber et al. 2015). Similarly, studies find increases in plant productivity and C stocks near the forest edge relative to the interior (Remy et al. 2016; Smith et al. 2018). Meeussen et al. (2021) found that aboveground biomass C and stem density were higher near the edge in temperate forests of Europe but that this did not translate to higher mineral soil C.
There are relatively few studies that quantify the belowground consequences of forest edge transitions. Notable exceptions include studies of total soil respiration across the northeastern US. Most observations from rural areas found that soil respiration was higher at the edge (Smith et al. 2019; Reinmann et al. 2020), though in some instances an increase in C loss from soil respiration was accompanied by an even larger increase in C gain from faster aboveground woody biomass accumulation (Reinmann et al. 2020). In these cases, enhanced soil respiration was attributed to higher temperatures at the edge (Smith et al. 2019; Garvey et al. 2022, 2023). However, the opposite is true for urban forests where edges have lower soil respiration than adjacent interior forests and compared to rural forest edges. Garvey et al. (2022) observed that forest edges in urban locations are, on average, drier and warmer than rural edges, suggesting that the much warmer temperature combined with a lack of soil moisture may limit soil respiration. Smith et al. (2019) measured soil respiration at the same site as this study and found that, consistent with other literature, soil respiration was higher at the edge. However, it remains unclear to what extent trends in soil respiration at forest edges are driven by changes in micro-meteorological conditions at the edge, increases in plant productivity above or belowground, or other factors.
Soil respiration includes both root (autotrophic) and microbial (heterotrophic) respiration, which may respond differently to the environmental gradient of a forest edge to interior. It is not only C pools and respiration that may change along such a transect, but also seasonal dynamics of plant and microbial activities that contribute to the overall C balance of the forest, such as root growth and mortality, soil decomposition by extracellular enzymes, and substrate availability. Each soil biogeochemical process can be affected in different ways by environmental conditions, and together their responses contribute to trends in ecosystem productivity and respiration rates observed at the site scale. In this study, our primary objectives were to (1) assess how changes in soil edaphic conditions at the forest edge influence, or are influenced by, soil biotic components, (2) determine if soil C pools and fluxes are higher at the edge relative to other landscape positions, and (3) understand how specific belowground processes contribute to bulk differences in pools and fluxes.
Methods
To better understand belowground responses across forest edges, we measured environmental conditions, live and dead fine root structural traits and biomass, soil chemistry, and soil respiration along a 75 m transect from forest to meadow. We made these measurements in a well-established urban forest edge-to-interior gradient in the Washington DC/Baltimore area that has been previously studied for total ecosystem C fluxes (Winbourne et al. 2022) between the forest and adjacent grassland.
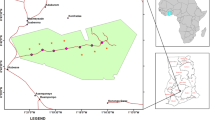
Site
We conducted this study at the National Institute of Standards and Technology (NIST) FOREST site ( Forested Optical Reference for Evaluating Sensor Technology). The FOREST site is a 26 ha forest fragment adjacent to an unmanaged grassland on the campus of NIST in Gaithersburg, Maryland (39°07′36.0"N 77°13′15.0"W). Within this forest patch is a one hectare plot (100 m by 100 m) that was established in 2016 to provide improved estimates of C fluxes from fragmented urban landscapes (Fig. 1). Previous work on this site includes determining edge effects on soil respiration rates (Smith et al. 2019), optical imaging through ground-based remote sensing techniques (Marrs et al. 2021), and a detailed estimate of site-scale biogenic CO2 fluxes (Winbourne et al. 2020). The site is a temperate broadleaf deciduous forest dominated by Liriodendron tulipifera, and Quercus sp. trees. The forest has a 3-m wide stream flowing through the plot approximately 25 m from the forest edge. The soil is a Glenelg series silt loam with a high clay content and low levels of soil organic matter (Natural Resources Conservation Service, 2018 from Smith et al. 2019). Mean summer (June, July and August) and mean winter (December, January and February) air temperatures are 23.5 °C and 1 °C, respectively (Smith et al. 2019). The annual mean precipitation is 1067 mm per year (National Climatic Data Center 2018).
In 2019, we established a new set of experiments at the FOREST site to characterize belowground processes across the forest edge gradient. This included soil and root sampling and additional soil water content and temperature monitoring along three central 75 m long transects (at 25 m, 50 m, and 75 m from the southern corner along the X direction; blue and pink circle symbols in Fig. 1). The transects extend from an open meadow into the forest interior from the southeastern aspect. There were six sampling locations along each transect. Each transect was representative of a landscape gradient along the Y direction beginning with two sampling locations in the meadow (-25 m, -12.5 m), one at the edge (0 m), and three in the forest interior (12.5 m, 25 m, 50 m). Landscape positions in meters refer to the distance from the edge (0 m), with positive distance in the direction of the forest interior and negative distance in the direction of the meadow (see grid in Fig. 1). In our study we additionally leverage existing study data collected across the site on soil respiration (shown as the yellow stars in Fig. 1). Winbourne et al. (2022) also noted a high density of deer in the area and the potential for their activity to affect understory vegetation productivity and maintain the open meadow. It is further important to note that the study design is pseudo-replicated in two ways, 1) sample locations are not fully independent due to their proximity in space, and 2) not all data types are replicated. The choice to use a regression framework, which maximizes sample locations at unique landscape positions over replication, was made so that it would be possible to consider distance to forest edge as a continuous variable in analyses and resolve nonlinear and nonmonotonic edge effects.
Environmental measurements
Vertical patterns of soil volumetric water content and temperature were assessed using frequency domain capacitance probes (Drill & Drop Probe, Sentek, Stepney, South Australia),Footnote 1 which measured volumetric water content (cm3 cm−3) and temperature (°C) every 10 cm depth to either 90 cm depth (-25, -12.5, 0, 12.5 m landscape positions) or 120 cm (25, 40 m landscape positions) for all three X landscape positions (25, 50, 75 m) (blue circle symbols in Fig. 1). For only the ridge locations, the probes were installed at 40 m along the transect point because the cables could not reach the 50 m point. On the probes are point measurements of temperature and an integrated 10 cm vertical measurement of water content. To install the probes, a battery powered or electric drill (using a portable generator) was used to drill holes to 90 or 120 cm depths, and the multi-sensor probes were then installed vertically until flush with the soil surface into the predrilled holes. Data was collected every 15 min from July 2019 to September 2021 and stored on a datalogger powered by solar panels. For seasonal and depth-profile analysis of soil temperature and moisture, 3-hourly data were used. Measurements of leaf area index were used in the random forest analysis (Sect. 2.3.3) as a proxy for aboveground productivity; these data are publicly available from Winbourne et al. (2021).
Soil and fine root measurements
Soil characterization and potential enzyme activity
In March, May and July of 2019, soil cores were collected using a core sleeve (15 cm height × 5 cm diameter) to 30 cm depth. The 0 cm to 15 cm depth soil cores were split again to create two soil subsample cores (0 cm to 7.5 cm, 7.5 cm to 15 cm). Samples were deposited into plastic bags and shipped on ice to Oak Ridge National Laboratory (ORNL) and stored at 4 °C until analysis. In the laboratory, we removed rocks and occasional large root fragments and determined their volume using water displacement. We measured fresh soil mass and then dried the soil at 70 °C for three days. Bulk density was calculated as dry mass divided by core volume and calculated gravimetric water content (GWC) as fresh mass minus dry mass divided by dry mass.
Soil subsamples were subsequently analyzed for pH in water and potential enzyme activity. Soil pH was determined by shaking 1 part soil in 2 parts deionized water and measuring the pH of the supernatant using a pH probe. Potential enzyme activity for seven different enzymes (β-D-cellobiosidase, α-glucosidase, β-glucosidase, N-acetyl-β-glucosaminidase, phosphomonoesterase, phosphodiesterase, β-xylosidase) was assessed by measuring the fluorescence produced by degradation of a substrate with a synthetic fluorescent indicator (4-methylumbelliferone) using a microplate reader (BioTec Synergy™MX Multi-Mode Microplate Reader.; Saiya-Cork et al. 2002; Steinweg et al. 2013). For the lab analysis, two replicates were used for analysis of nitrogen (N) (%), C (%), C:N, soil pH, and GWC (%). Three replicates were used for the potential enzyme activity analysis. Total enzyme activity was calculated by summing enzyme activity for the seven enzymes measured in this study.
We determined soil particle size fractions (sand, silt and clay) using the Bouyoucos hydrometer method on oven-dried and ground samples from March sampling (Gee and Or 2002). Chloroform fumigation was used to determine microbial biomass C (MBC), N (MBN), and phosphorus (MBP) (Vance et al. 1987). Samples were analyzed for total dissolved organic carbon (DOC) and dissolved nitrogen (DN) using a Shimadzu total organic C analyzer (Shimadzu TOC-L CSH/CSN analyzer, Baltimore, MD, USA). Total C and N analyses were performed on air dried, ground soil using a LECO TruSpec CN analyzer (LECO Corp., St. Joseph, MI). Samples were analyzed for dissolved phosphorus (DP), and extractable ammonium (NH4) and nitrate (NO3) using high-performance liquid chromatography (Dionex ICS-5000 + Thermo-Fisher Waltham, MA, USA) with the Dionex IonPac AS11-HC column using a potassium hydroxide eluent and gradient elution. Total bulk soil phosphorus (P) was determined using a Flow Injection Analyzer based on the Lachat Method No 13–115-01–1-B at the University of Missouri Soil and Plant Diagnostic Laboratories.
Fine root processing
From the soil cores collected in March, May, and July of 2019 (as described in Sect. 2.3.1), fine root biomass and properties were determined. Fine roots < 2 mm (primarily consisting of root orders 1–3) were floated in distilled water and manually removed from soil using tweezers, then separated by plant functional type (grass or tree species) and if they were dead or alive. Fine roots were characterized as woody tree roots due to the presence of bark and a circular, obvious cortex, as well as being darker and larger in diameter with more pronounced first order roots compared to grassy roots. Roots characterized as grasses were stringy, light in color, smooth in texture, appearing homogeneous throughout (no obvious cortex), and relatively longer than tree roots. Dead fine roots were determined by being easily broken apart often with signs of decay, and/or having soft tissue that was easily deformed when pushed with tweezers. Each fine root class was then placed into clear plastic trays filled with water and displayed to minimize overlap, which was then scanned using a photo scanner (Epson V700). Root traits (fine root length, fine root diameter) were then assessed using software (WinRhizo). Finally, fine roots were dried at 70 °C to constant weight to obtain dry fine root biomass (g) values. In this study, we focused on fine roots rather than all roots (including live and dead coarse roots), in part due to limited capacity for destructive sampling but primarily because fine roots constitute the vast majority of the highly absorptive, functional root surface area and interface for competitive resource uptake (Pregitzer et al. 2002, McCormack et al. 2015, Warren et al. 2015).
Respiration and trenching
Our study leverages existing study design at the FOREST site of ongoing soil respiration measurements along the forest edge transect. As described in Smith et al. 2019, in 2016 thirty-three pairs of 15.5 cm diameter × 7 cm tall polyvinyl chloride soil collars were placed 10 m apart at six distances (0 m, 12.5 m, 25 m, 50 m, 75 m and 100 m; see Fig. S1 in Smith et al. 2019) in the X and Y direction from the forest edge. Five collars that were 125 m away from the forest edge in either the X or Y direction were later added (Fig. 1). In March of 2019, a trenching experiment was established at the site to partition soil respiration fluxes into the autotrophic and heterotrophic components. For 11 of the existing soil respiration collars, a 1 m × 1 m × 0.5 m deep trench was excavated around each collar. Trenching was achieved using a 15.24 cm (6 in) wide trenching attachment on a wheeled bobcat tractor. To protect the ground near the collars from compaction, 122 cm (4 ft) × 244 cm (8 ft) sheets of plywood were placed around the trenched areas to distribute the weight of the equipment. To limit root and fungal hyphal connections between the trenched plot and the surrounding soil, each trench was lined with a 1 μm nylon mesh fabric (Sefar Nitex 3A03-0001–115-00, Elko Filtering Co.). The fabric was tacked into the upper soil surface using landscape garden stakes and the trench was repacked with soil. In all cases, respiration collars were placed in the center of trenched plots to avoid potential edge effects where trenching occurred. Within 10 m of all trenched plots remain the paired, untrenched soil respiration collars. Of the 11 trenched plots, three are at the edge position, two are in the forest interior at 12.5 m from the edge, three are in the interior at 75 m, and three are in the meadow position where additional soil collars were installed within and outside of the trenched plots. At 12.5 m from the edge, rocks at depth and the stream prevented installation of the third replicate trench plot, and sloping soil at 25—50 m prevented installation at these locations entirely (Fig. 1).
Measurements of soil CO2 efflux were collected roughly every two weeks through the growing seasons from June 2, 2017 to February 27, 2020. Measurements from trenched and untrenched collars were collected with the same frequency in 2019 (May 23, 2019 to September 26, 2019), with an additional measurement made at the end of February 2020 (February 27, 2020). Total soil respiration was measured from the CO2 flux from all untrenched collars using a gas exchange system (LI-COR LI-840A) following the vented chamber design described by Savage and Davidson (2001). Additional collars were placed in trenched plots (n = 11) to capture heterotrophic respiration. Autotrophic respiration was estimated by subtracting the respiration rate measurement made at each trenched collar from the measurement at its paired untrenched collar. In some cases where the trenched plot was near more than one representative untrenched soil respiration collar, respiration fluxes from multiple untrenched collars were averaged before calculating autotrophic respiration by difference for each measurement period. The measurement period was within the same day for the majority of measurements. However; in some cases where fluxes for the paired collar were measured the following day we considered the two-day period as a measurement period. Paired collars are indicated by a pairs ID column in the soil respiration data product linked to this paper (see Data Availability).
Analysis
Root and soil measurements originating from the meadow (-25 m, -12.5 m), edge (0 m), and forest interior (12.5 m, 25 m, 50 m) were binned together and pair-wise t-tests used to determine whether variables measured in the meadow or the forest interior were different from the edge. Linear regression was used to determine the relationship between the fraction of heterotrophic respiration and environmental variables. We examined the presence of edge effects in aboveground biomass, aboveground biomass increment, and stem density using published data from Winbourne et al (2020) that is publicly available at https://doi.org/https://doi.org/10.7910/DVN/UNF7EB. This included a diameter at breast height (DBH) resurvey that was conducted in 2017 and again in 2019.
To understand which environmental variables most influence soil respiration without assuming that relationships between all variables are linear, we fit a Random Forest machine learning model to the N = 763 soil respiration measurements with paired environmental information. We fit this model using the ranger and caret packages in R (Version 0.14.1). We set the minimum node size to 5, mtry to the rounded down square-root of the number of predictor variables, and grew 1000 trees for each model. We started with all environmental variables in our dataset present in the model and removed variables with lowest importance ranking until highest R2 was reached. We evaluated model performance using tenfold cross validation, holding out 20% of the data each time for testing. The final model, fit to soil respiration as the dependent variable, had an R2 = 0.66, MSE = 0.51, and N = 763.
Results
Environmental conditions
Soil at the forest edge was drier than the meadow and the forest interior, especially at middle depths (e.g., 25 cm to 85 cm) and during late-summer dry-down periods (Figs. 2 and 3). Meadow and interior positions had a smaller range of observed soil moisture values throughout the year, with neither landscape position drying down to less than 20% soil moisture at any time. In contrast, the forest edge had a minimum value of 14% at 15 cm depth and 18% at 75 cm depth. The meadow was consistently warmer and wetter than the edge or interior positions, regardless of season (Figs. 2 and 3).
Fine root traits and aboveground biomass
As expected, live fine root biomass of grass roots was highest in the meadow and tree roots in the forest interior (Fig. 4). The forest edge and interior did not have significantly different live fine root biomass. Dead fine root biomass in grasses was also highest in the meadow, with no difference between the forest edge and interior. However, dead tree fine root biomass was significantly higher at the forest edge than at the other landscape positions (i.e., pair-wise tests between the edge and both the meadow and forest interior were different at the P < 0.05 level; Fig. 4b right panel), followed by the forest interior and then the meadow. We also measured other fine root traits, including live and dead fine root length and fine root length per unit soil volume, but here we observed what we would typically expect: higher grass root length in the meadow and higher tree root length in the interior, and the forest edge in all cases was not significantly different from the forest interior.
Boxplots of (a) live and (b) dead fine root biomass for each landscape position. Text indicates the P-value for a one-way ANOVA of landscape position ~ root variable. Symbols at the top of the graph indicate whether pair-wise t-tests of difference from the edge position are not significant (“ns”), significant at the P ≤ 0.05 (*), P ≤ 0.01 (**), P ≤ 0.001 (***), or P ≤ 0.0001 (****)
The aboveground biomass, aboveground biomass increment, and stem density were higher (1.5, 1.4, and 1.8 times greater, respectively) within 12.5 m of the forest edge than adjacent interior forest. Aboveground biomass and stem density were also higher 20 m from forest edge than the forest interior, although this was no longer the case at 25 m from the edge (Table S1).
Soil properties
We observed strong meadow-to-interior gradients for pH and C:N. pH was more acidic in the forest interior, and C:N was higher in the forest interior (Fig. 5a,b). The forest edge was a strong inflection point for both of these properties (Fig. S1). There was a slight trend towards higher α-glucosidase (AG, sugar degradation) at the edge (Fig. 5c). There were also slight trends towards higher DOC, dissolved phosphorus (DP), and microbial biomass phosphorus (MBP) in the forest interior, with similar but more statistically marginal differences in phosphodiesterase (DPS), β-xylosidase (XYL), microbial biomass carbon (MBC), and phosphomonoesterase (PS) (Fig. S2). Other measured values, including microbial biomass N concentration (MBN), β-glucosidase (BG), N-acetyl-β-glucosaminidase (NAG), NH4, NO3, and total bulk soil P were not different across landscape positions. Total enzyme activity tended to be higher in the forest interior, although this pattern varied depending on the depth and time of year, with lower enzyme activity in the forest interior at shallow depths (0 cm to 15 cm) during the summer (Fig. S3). Lastly, differences between soil C content were only marginally significant (Fig. 5d). The soil C content of both the meadow and interior were not significantly different from the edge by 1-way ANOVA; however, soil C content in the meadow was significantly lower than in the forest interior at the P ≤ 0.05 level. Although median soil C content tended to be higher in the forest interior, average soil C content was higher at the edge.
Boxplots of (a) pH, (b) C:N, (c) sugar degradation (α-glucosidase; AG), and (d) soil C content (%) for each landscape position. Text indicates the P-value for a one-way ANOVA of landscape position ~ soil variable. Symbols at the top of the graph indicate whether pair-wise t-tests of difference from the edge position are not significant (“ns”), significant at the P ≤ 0.05 (*), P ≤ 0.01 (**), P ≤ 0.001 (***), or P ≤ 0.0001 (****)
Soil respiration and partitioning
Soil and heterotrophic respiration were significantly higher at the forest edge compared to the interior (Fig. 6). Compared to the meadow, autotrophic respiration was significantly higher at the forest edge, while heterotrophic respiration was significantly lower (Fig. 6). There was a clear negative relationship between the fraction of total soil respiration which was heterotrophic and volumetric soil moisture (Fig. 7, Fhet ~ VSM, F1,72 = 39.07, R2 = 0.34, P < 0.0001), but there was a much weaker relationship between the fraction of heterotrophic respiration and soil temperature (Fhet ~ Soil Temperature, F1,89 = 4.96, R2 = 0.04, P = 0.03).
Boxplots of soil respiration separated into autotrophic respiration (Ra), heterotrophic respiration (Rh) and total soil respiration (Rs) for each landscape position. Text indicates the P-value for a one-way ANOVA of landscape position ~ soil variable. Symbols at the top of the graph indicate whether pair-wise t-tests of difference from the edge position are not significant (“ns”), significant at the P ≤ 0.05 (*), P ≤ 0.01 (**), P ≤ 0.001 (***), or P ≤ 0.0001 (****)
Relationship between the fraction of soil respiration that was heterotrophic and the volumetric soil moisture (VSM) in (%). Some values are higher than one when the heterotrophic respiration measured in the trenched plot was higher than the untrenched plot, likely indicating additional heterotrophic respiration from decomposing roots that were severed during the trenching treatment
Trenching methods can bias estimates of autotrophic and heterotrophic respiration in systematic ways (Savage et al. 2018). Plant roots severed by trenching decompose in place and can therefore increase estimates of heterotrophic respiration relative to autotrophic respiration. In the meadow position, trenching severed woody roots but did not necessarily exclude shallow-rooted grasses, such that heterotrophic respiration in the meadow may have included autotrophic respiration from grasses. Because the meadow position had fewer woody roots than the forest edge and interior (Fig. 4, right panels), we expect less of an effect of decaying roots, but more of an effect from the incomplete exclusion of grass.
Lastly, a Random Forest model using environmental predictors to estimate soil respiration showed that soil temperature was the most important environmental variable explaining the soil respiration rate, followed by time information, volumetric soil moisture, leaf area index (LAI), and landscape position (Fig. 8).
Discussion
We observed many differences in the belowground environment between the forest interior, edge and adjacent meadow. In some cases, the forest edge represented a mid-point in environmental or belowground characteristics between the forest interior and meadow (e.g., pH, C:N, live fine root biomass), likely reflecting the change in litter type and quality associated with the transition from grass to woody species. In other cases we observed environmental and belowground conditions at the forest edge that were different from the other landscape positions. For example, forest edge soil was drier than both meadow and forest interior soil (Figs. 2 and 3). This observation is consistent with other observations of rural and urban forest edges (Garvey et al. 2022, Crockatt and Bebber 2015), but often co-occurs with and is attributed to higher soil temperatures. We did not observe higher soil temperature at the edge in our study, suggesting that the dry forest edge was due to other factors. Forest edges commonly have increased plant productivity and/or stem density (Morreale et al. 2021 Nature, Reinmann and Hutyra 2017), both of which were true at our site in the first 12.5 m of the forest edge (Table S1). This likely resulted in increased water demand, evapotranspiration and subsequent soil moisture drawdown, providing a potential explanation for the low soil moisture observed at the edge (Reinmann et al. 2020).
Dead fine root biomass was also higher at the forest edge, implying that forest edge roots experienced higher rates of mortality, lower rates of decomposition, or both. Both could be explained by drier soils at the forest edge. It is not clear that dead fine root biomass was contributing to either faster or slower decomposition, however, as most measurements of potential enzyme activity were either not different between landscape positions or the edge was intermediate between meadow and interior. One exception was α-glucosidase, responsible for sugar degradation, which was slightly higher at the forest edge (Fig. 5c).
There was a strong meadow-to-forest gradient in another measure of decomposition, soil respiration. Lower respiration was consistently observed in the forest interior, but soil C contents were not significantly different across meadow, edge, and interior (P = 0.06). Soil respiration partitioning also depended on landscape position, with higher heterotrophic respiration in the meadow relative to the forest interior. Overall soil respiration was most influenced by soil temperature (Fig. 8), but the partitioning between autotrophic and heterotrophic respiration was most responsive to volumetric soil moisture (Fig. 7). There were obvious differences in plant type and soil moisture between landscape positions, which may interact to explain differences in heterotrophic respiration due to landscape position. For example, the meadow position was warmer than forest edge and interior, which may explain the high soil heterotrophic respiration in the meadow (Figs. 2 and 3). The forest edge was not warmer than the interior but still had significantly higher soil and heterotrophic respiration, possibly due to the large dead root pool at the edge stimulating microbial activity.
Average soil C content was marginally higher at the edge relative to other landscape positions (Fig. 5d). There are a wide range of potential processes that would result in both higher soil respiration and soil carbon content at the edge compared to the forest interior. For example, increased plant productivity at the edge could result in more autotrophic respiration from live roots, more heterotrophic respiration from decomposition of dead roots, or both. We were not able to observe significant differences in live root biomass, but this could be due to sample size and spatial heterogeneity rather than a true lack of effect. The same increase in productivity could result in a larger pool of dead roots, the decay of which is a direct input to the soil C pool. It is not clear however, if the larger pool of dead roots, an acceleration of decomposition rates, or both, is occurring at this site.
In order to test the first two hypotheses, i.e., autotrophic respiration and/or heterotrophic respiration is higher at the edge compared to forest interior, additional measurements of respiration partitioning are needed, combined with sampling of total root biomass, including coarse roots. Future studies addressing this could help clarify whether total live root biomass differs across landscape positions. Measurements of specific root respiration could be used to distinguish whether root respiration per unit biomass differs across the gradient. In order to test the second pair of hypotheses, i.e., larger pool of dead roots and/or faster decay of dead roots at the edge, incubation and litterbag experiments to observe the rate of dead root decomposition across landscape positions could be used. The higher rates of potential sugar degradation observed at the edge compared to forest interior suggest that dead roots may decompose faster at the edge, but these measurements are potential activity rates, and may not represent field rates, particularly under the drier conditions that we observed at the forest edge.
There are a number of factors in urban forest fragmentation studies that limit the ability to generalize results from one study to a region or more widely. First, there was extensive deer browsing in the area around our plots, potentially changing both plant and nutrient inputs to soil compared to a forest without browsing activity. Because we did not remove any grasses in the trenched collars, we are limited in our interpretation of respiration partitioning in the meadow landscape positions. The trenching method itself also suffers from artifacts such as decomposition of recently severed roots, that may result in an overall overestimation of heterotrophic respiration (Savage et al. 2018). Lastly, the measurement period was reduced from the original sampling plan due to the COVID-19 pandemic, so that for many measurements of soil and root characteristics, only one year of seasonal data were collected. Another general caveat is that the response of forests to fragmentation can vary greatly depending on the biome and ecosystem, as well as land use history. For example, temperate broadleaf regions like the site of this study commonly find increased biomass and productivity at forest edges, while the opposite tends to be true in tropical and boreal forests (Smith et al. 2018; Meeussen et al. 2021).
Forest fragmentation has important biogeochemical consequences beyond the more easily measured changes in aboveground plant composition, structure, and productivity. In particular, we show here that there are substantial gradients in belowground biogeochemical processes, including fine root traits, soil chemistry, and microbial activity. Based on this study and others in the region, temperate broadleaf forests seem to have consistently drier edges with higher soil and heterotrophic respiration than the forest interior. Here, we show that the forest edge had higher dead tree fine root biomass and sugar degradation, suggesting higher rates of root growth, mortality, and subsequent decay at forest edges. Soil C contents were only marginally different across meadow, edge, and interior, suggesting an overall acceleration of carbon cycling at the edge, without changing belowground carbon concentration. However, for a more complete understanding of carbon cycling across this forest fragment, a detailed carbon budget is needed. In addition to understanding the plant and soil C consequences of forest fragmentation and other human management, it is also critical to consider ecosystem services other than C, including water storage and filtration, wildlife habitat, traditional land uses, and local climate regulation.
Data Availability
The data used in this manuscript is publicly available and can be accessed at https://tes-sfa.ornl.gov/node/80 or https://doi.org/10.25581/ornlsfa.024/1837084.
Notes
Certain equipment, instruments, software, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement of any product or service by NIST, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
References
Briber BM, Hutyra LR, Reinmann AB et al (2015) Tree productivity enhanced with conversion from forest to urban land covers. PLoS ONE 10:1–19. https://doi.org/10.1371/journal.pone.0136237
Crockatt ME, Bebber DP (2015) Edge effects on moisture reduce wood decomposition rate in a temperate forest. Glob Chang Biol 21:698–707. https://doi.org/10.1111/gcb.12676
Franklin CMA, Harper KA, Clarke MJ (2021) Trends in studies of edge influence on vegetation at humancreated and natural forest edges across time and space. Can J for Res 51:274–282. https://doi.org/10.1139/cjfr-2020-0308
Garvey SM, Templer PH, Bhatnagar JM, Hutyra LR (2023) Soils at the temperate forest edge: an investigation of soil characteristics and carbon dynamics
Garvey SM, Templer PH, Pierce EA, et al (2022) Diverging patterns at the forest edge : Soil respiration dynamics of fragmented forests in urban and rural areas. 3094–3109. https://doi.org/10.1111/gcb.16099
Gee GW, Or D (2002) 2.4 Particle‐size analysis. Methods soil Anal Part 4 Phys methods 5:255–293
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci Adv 1:1–10. https://doi.org/10.1126/sciadv.1500052
McCormack ML, Dickie IA, Eissenstat DM et al (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518
Meeussen C, Govaert S, Vanneste T et al (2021) Drivers of carbon stocks in forest edges across Europe. Sci Total Environ 759:143497. https://doi.org/10.1016/j.scitotenv.2020.143497
Morreale LL, Thompson JR, Tang X et al (2021) Elevated growth and biomass along temperate forest edges. Nat Commun. https://doi.org/10.1038/s41467-021-27373-7
National Climatic Data Center (2018) Climate data online: Data tools. Retrieved from www.ncdc.noaa.gov/cdo-web/datatools/normals
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine north American trees. Ecol Monogr 72:293–309. https://doi.org/10.1890/0012-9615(2002)072[0293:FRAONN]2.0.CO;2
Reinmann AB, Hutyra LR (2017) Reply to Remy et al.: Local and global limitations to forest productivity as mediators of biogeochemical response to forest edge effects. Proc Natl Acad Sci U S A 114:E7033–E7034. https://doi.org/10.1073/pnas.1712103114
Reinmann AB, Smith IA, Thompson JR, Hutyra LR (2020) Urbanization and fragmentation mediate temperate forest carbon cycle response to climate. Environ Res Lett. https://doi.org/10.1088/1748-9326/abbf16
Remy E, Wuyts K, Boeckx P et al (2016) Strong gradients in nitrogen and carbon stocks at temperate forest edges. For Ecol Manage 376:45–58. https://doi.org/10.1016/j.foreco.2016.05.040
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Savage KE, Davidson EA (2001) Interannual variation of soil respiration in two New England forests. Global Biogeochem Cycles 15:337–350. https://doi.org/10.1029/1999GB001248
Savage KE, Davidson EA, Abramoff RZ et al (2018) Partitioning soil respiration : quantifying the artifacts of the trenching method. Biogeochemistry. https://doi.org/10.1007/s10533-018-0472-8
Smith IA, Hutyra LR, Reinmann AB et al (2018) Piecing together the fragments: elucidating edge effects on forest carbon dynamics. Front Ecol Environ 16:213–221. https://doi.org/10.1002/fee.1793
Smith IA, Hutyra LR, Reinmann AB et al (2019) Evidence for Edge Enhancements of Soil Respiration in Temperate Forests. Geophys Res Lett 46:4278–4287. https://doi.org/10.1029/2019GL082459
Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Official Soil Series Descriptions. Available online. Accessed 2018
Steinweg JM, Jagadamma S, Frerichs J, Mayes MA (2013) Activation energy of extracellular enzymes in a global suite of soils. PLoS ONE 8(3):e59943. https://doi.org/10.1371/journal.pone.0059943
Thompson JR, Carpenter DN, Cogbill C V, Foster DR (2013) Four centuries of change in northeastern United States forests. PLoS One. https://doi.org/10.1371/journal.pone.0072540
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass – C. Soil Biol Biochem 19:703–707
Warren JM, Hanson PJ, Iversen CM et al (2015) Root structural and functional dynamics in terrestrial biosphere models–evaluation and recommendations. New Phytol 205:59–78
Winbourne JB, Jones TS, Garvey SM et al (2020) Tree transpiration and urban temperatures: current understanding, implications, and future research directions. Bioscience 70:576–588
WinbourneJB (2021) Quantification of urban forest and grassland carbon fluxes using field measurements and a satellite-based model in Washington DC/Baltimore area. Harvard Dataverse, V3. https://doi.org/10.7910/DVN/UNF7EB
Winbourne JB, Smith IA, Stoynova H et al (2022) Quantification of Urban Forest and Grassland Carbon Fluxes Using Field Measurements and a Satellite-Based Model in Washington DC/Baltimore Area. J Geophys Res Biogeosciences. https://doi.org/10.1029/2021JG006568
Zuidema PA, Sayer JA, Dijkman W (1996) Forest fragmentation and biodiversity: The case for intermediate-sized conservation areas. Environ Conserv 23:290–297. https://doi.org/10.1017/s037689290003914x
Acknowledgements
This research was sponsored by the U.S. Department of Energy (DOE) Office of Biological and Environmental Research program. Oak Ridge National Laboratory (ORNL) is managed by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the U.S. DOE. RZA also thanks the Sitka Center for Art and Ecology.
Funding
This research was sponsored by the U.S. Department of Energy (DOE) Office of Biological and Environmental Research program. Oak Ridge National Laboratory (ORNL) is managed by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the U.S. DOE.
Author information
Authors and Affiliations
Contributions
The study was designed by JMW, JW, AR, LH, and MAM. JMW, JH, SO, JRP, JW, IS, AR, LH, DWA, and MAM contributed to material preparation and data collection. Analysis was conducted by RZA, JW, JMW, JH, LH, SG and MAM. LH, AR, and MAM contributed to funding acquisition. The first full draft of the manuscript was written by RZA and all authors participated in writing and revision.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This manuscript has been authored by UT-Battelle, LLC under Contract No. DE-AC05-00OR22725 with the U.S. Department of Energy. The United States Government retains and the publisher, by accepting the article for publication, acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. The Department of Energy will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abramoff, R.Z., Warren, J.M., Harris, J. et al. Shifts in belowground processes along a temperate forest edge. Landsc Ecol 39, 100 (2024). https://doi.org/10.1007/s10980-024-01891-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10980-024-01891-3