Abstract
Context
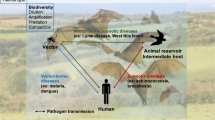
Vector-borne diseases (VBD) are a worldwide public health problem for humans and wildlife. 'Global Change' such as habitat alteration and land-use intensification, biotic exchange, the introduction of invasive alien species, and climate change have all been linked to an increased occurrence of VBDs.
Objectives
To evaluate the impact of land-use and land-cover (LULC) on the transmission of VBDs, we conducted a systematic review of the existing literature on the global effects of land use on VBDs. This was followed by a meta-analysis to test the relationship between LULC intensification and infection prevalence.
Methods
Overall, 654 articles met our inclusion criteria for the systematic literature review, and 18 studies fulfilled the requirements for the meta-analysis.
Results
The systematic literature review identified 162 articles with a total of 2541 data entries on the effect of LULC on VBDs. The majority of the studies were published after 2010, and the spatial distribution of data was biased towards North America and Europe. Overall, 193 different hosts and 144 different vector groups were identified. Avian and human malaria were the most frequently named diseases, with humans and Anopheles mosquitoes the most common host and vector, respectively. Our meta-analysis found that land-use intensity, as well as targets (host and vector), significantly impact the prevalence of VBDs. Tests for both residual heterogeneity and moderators were significant, where lower land-use intensity and vectors were linked to lower VBD prevalence, while medium land-use intensity was associated with higher prevalence. Analysis of the host sub-model supported these findings, with infection prevalence significantly lower in low land-use intensity.
Conclusions
The systematic literature review revealed a temporal increase in publications on this topic, with a significant rise since 2007 and uneven distribution of data across countries, with the United States, Spain, and Brazil being the most prominent contributors and identified a wide range of pathogens and hosts involved in VBD systems, with human and avian malaria being the most commonly mentioned diseases. We also show, through a meta-analysis, that LULC intensification affects VBDs infection prevalence. Future studies should incorporate the effects of land-use intensity on vector-borne diseases in diverse ecosystems to inform management strategies and mitigate disease emergence with implications for human, livestock and wildlife health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vector-borne diseases (VBDs) are infectious diseases that are transmitted to humans or wildlife (the hosts) through blood-feeding arthropods (the vectors), such as mosquitoes, ticks, or fleas. They have long been recognized as a significant public health concern worldwide with major economic implications (Kilpatrick and Randolph 2012; Franklinos et al. 2019).
Humans and wildlife are at risk of VBD, with globally over 80% of the human population threatened by infections (Kilpatrick and Randolph 2012; Franklinos et al. 2019). The global burden of VBD is overwhelming, with more than 580 million reported cases and over one million reported deaths annually worldwide (WHO and UNICEF 2017). It is generally accepted that VBDs are on the rise, driven by climate change (Altizer et al. 2006; Pérez-Rodríguez et al. 2013a), although optimal temperature range varies depending upon the vector and/or pathogen (e.g., for mosquito-borne pathogens). While the impact of climate change on VBDs has been well-studied, the effects of land-use/land cover (LULC) on disease dynamics are little understood (Franklinos et al. 2019), even though it has an unprecedented impact on global biodiversity and the environment (Sala et al. 2000; Rohr et al. 2020). This is also one of the key points highlighted recently in the “Report of the scientific task force on preventing pandemics” (Alimi et al. 2021).
Unfortunately, the magnitude of the VBD problem in general has only recently become apparent, especially highlighted by the United States, with a doubling of the reported numbers of VBDs between 2004 and 2016 (Rosenberg et al. 2018). Globally, mosquito and tick-borne diseases are increasing their incidence and geographic distribution, thus affecting new areas (Stanaway et al. 2016; Gilbert 2021) and re-emerging in regions from which they had previously been eradicated. Indeed, mosquitoes, with over 3,500 species worldwide, are by far the most common vector of pathogens (Tolle 2009), not only responsible for the transmission of Plasmodium (malaria), dengue virus and West Nile virus, among numerous others, but also for emerging and re-emerging diseases such as chikungunya and zika (Tolle 2009). Dengue and malaria have no affordable and effective vaccine yet, but are affecting up to 450 million persons per year (Franklinos et al. 2019). However, mosquitoes are not the only important vectors, and in the last decade there has been a significant increase of tick-borne infectious diseases. Some of these diseases, for example the Crimean-Congo haemorrhagic fever, have very high mortality rates in humans of up to 50% (Ergönül 2006; Beaute et al. 2018).
Diverse environmental factors can influence the transmission and incidence of VBDs by directly affecting either the pathogen transmission or vector behaviour. These factors can also have indirect effects on VBDs by altering the life cycles of vectors or reservoirs, which ultimately impacts their distribution and abundance (Caminade et al. 2019). Despite predicted impacts of LULC on biodiversity loss (Sala et al. 2000), there is, with the exception of several correlative studies and reviews, little evidence about how land-use intensification impacts VBDs and the transmission of pathogens. Previous research has focused on how global change, as climate variation or biodiversity loss in general, affects VBDs (e.g., Altizer et al. 2013), generally neglecting other potential drivers, such as LULC (Sala et al. 2000).
To address this knowledge gap, we (1) reviewed the existing literature on the transmission of VBDs globally and (2) analysed the factors related with global change that potentially affect transmission risk of VBDs, particularly discriminating between climate change and LULC effects. Therefore, we tested the reliability and availability of data related to VBDs and LULC, following a systematic review approach. Factors that could affect the VBD infection prevalence were then analysed using a meta-analysis approach. Infection prevalence refers to the proportion or percentage of individuals in a defined population who are infected with a specific pathogen at a given point in time (Jovani & Tella 2006). The ultimate target of pathogens, i.e., host versus vectors, were analysed separately as they may be mediated by land-use intensity differently, which in turn could affect transmission and infectivity. We expected that land-use intensification would affect host and vector prevalence differently, with an overall higher prevalence of VBDs in areas that are more intensely impacted by humans. Our results will help to recognize general patterns, if they exist, and we expect to advance knowledge of pathogen transmission dynamics by understanding the role of land-use intensity in detail.
Methods
The systematic literature search strategy
We performed a systematic review of literature using PubMed, Google Scholar and the Web of Science (WoS) Core Collection for all papers published until October 28th, 2019 (timespan: all years. Indexes: SCI-EXPANDED, SSCI, Current Contents Connect + Derwent Innovations Index + KCI—Korean Journal Database + MEDLINE® + Russian Science Citation Index + SciELO Citation Index + Zoological Record) to improve the understanding, scope, and outcome of LULC drivers on VBD studies. The search criteria and protocol are available in Appendix 1. This systematic review follows the standards of Preferred Reporting Items for Systematic reviews and Meta-analysis (PRISMA) 2020 guidelines (Page et al. 2021) (Appendix 2). Using explicit and systematic methods, all studies that were relevant to the relationship between LULC and VBDs were selected and critically evaluated. We included in the systematic review articles that referred to the presence of any VBD in relation to host and/or vector data and that contained information on LULC. In addition, when information on VBD prevalence was available, either in the hosts and/or in the vectors, data from these studies were collected and analysed. If this information was unavailable, but the number of infected individuals and the total number of subjects in the population were known, prevalence was manually calculated by dividing the number of infected individuals by the total number of subjects tested in each population.
A PRISMA flow diagram was created that specifies the selection procedure in detail, including all the steps carried out (Fig. 1), also indicating which number of articles were included or excluded and for what reasons. A peer-review was performed independently by three authors (MF, SM, SCR) in which each person read all the abstracts, followed the inclusion/exclusion criteria, and produced the final list of publications for reading the full text. This first filtering was carried out by responding to the following questions (search terms in Box 1, Appendix 1) about whether the study:
-
investigates any VBD at any place worldwide?
-
includes any LULC information related to infection prevalence in the hosts and/or vectors, or indicates the geographic coordinates of the sampling site to calculate the land-use intensity?
-
specifies the VBD vector and/or host species?
-
indicates the sample size (N)?
-
clearly lists the prevalence of infection in the hosts and/or vectors, or was the information inferable from the text, tables, or any supplementary material accessible, or indirectly deduced from figures?
-
clearly lists any error measure, such as standard deviation (S.D.), standard error (S.E.) or 95% confidence intervals (C.I.), or is this information inferable from the figures or tables?
Each reviewer read all the selected articles and entered the information of interest and the variables into a shared database, following the PRISMA flow diagram (Fig. 1). During the second filtering, all articles that did not meet the criteria of our objectives were excluded. Finally, tables were completed covering data from all the articles, which contained information on the:
-
(i)
main characteristics of the article (i.e., article ID, year of publication, year of sampling, information on the host and/or vector species and/or pathogen of the study, location/province/country/longitude-latitude, infection prevalence in the hosts and/or vectors, sampling size, any error measure),
-
(ii)
LULC classification, studies which describe associations with any simplified descriptors (e.g., urban, rural, natural); and,
-
(iii)
details about the kind of the study (e.g., review and/or modelling study on VBDs, any predictive, spatial, or epidemiological modelling, among other information potentially relevant for the study).
Characterisation of land-use intensity
A clear definition of land-use intensity is crucial in studies investigating VBDs (Lüdtke et al. 2013; Sehgal 2015; Renner et al. 2016). Although the reviewed articles did not explicitly provide data on land-use intensification, they presented information on interpreted changes associated with different land-use classes, indicating spatial rather than temporal changes. To avoid potential bias stemming from authors' decisions to provide or withhold unpublished data, we did not explicitly request such data.
To ensure comparable data, we manually assigned a value of land-use intensity using the "Habitat Classification Scheme" proposed by Jung et al. (2020), which aligns with the International Union for Conservation of Nature (IUCN) criteria (https://www.iucnredlist.org/resources/habitat-classification-scheme). This classification procedure was conducted following their interactive global habitat map with a resolution of approximately 100 m (Table 1). Land-use intensity was categorized into three classes: high (areas with continuous human presence and significant landscape alteration, such as artificial habitats), medium (areas with human presence but still retaining some extent of natural habitats, including a mixture of natural and artificial habitats), and low (areas where human presence is unlikely, and the landscape remains largely unaltered, such as natural habitats).
We adopted this classification procedure to ensure consistency in the assessment of LULC intensity across all analysed articles. While we acknowledge the potential limitations associated with the use of 2015 LULC data, it is important to note that our approach remains appropriate for our meta-analysis, given that the included studies were published, on average, in 2016. The effect size values extracted from the meta-analysis papers exhibit a temporal progression, with a mean value in 2015, further justifying the use of an LULC map corresponding to this specific time point. Our method primarily aims to assess the deceleration of land-use alteration observed globally between 2006 and 2019 (Winkler et al. 2021). The findings of Winkler et al. (2021) suggest that environmental conditions have remained relatively stable worldwide over the past two decades. Furthermore, to assess the consistency of the LULC classification, the Human Footprint Index (HFI) was calculated for the same sampling areas from the studies included in the meta-analysis (Mu et al. 2022). This metric quantifies the extent of human influence on the environment, taking into account factors such as population density, land use, and infrastructure development (see Appendix 3).
Meta-analysis procedure
Articles that met the criteria of specifying (1) infection prevalence in hosts or vectors per any LULC (if possible, we converted parasite intensity and parasitaemia into infection prevalence), (2) any measure of error (such as S.D., S.E. or 95% CI), (3) the sample size (N) and (4) any LULC class/type or land-use intensity that were named, quantified, or qualified were evaluated using a meta-analysis procedure.
The estimates of infection prevalence were calculated according to multiple combinations of the target (if the prevalence was described in the host or vector) and the established land-use intensity category (high, medium or low). To carry out the meta-analysis, we estimated the effect size the (yi) and sampling variance (vi) using the raw mean (MN) method, controlling for the S.D., sampling size and infection prevalence with the “escalc” function from the “metafor” package in R. To analyse the variability between studies, we assessed Higgins’ I2 and Cochran’s Q method (DerSimonian and Laird 1986). We used radial plots as a way to visually assess heterogeneity within the meta-analysis when outcomes had differing precisions due to heteroscedastic sampling variances (Galbraith 1994). Random-effect models were conducted using the estimate of heterogeneity as an inverse-variance model. We assessed publication bias using the Eggers test and visually inspected the funnel plots. The “trim and fill” method was used to estimate the importance of missing studies not included in the meta-analysis. A multivariate linear model was performed, including mixed-effects, based on a random-effects model with the "rma.mv" function. With this approach, we investigated factors potentially contributing to the heterogeneity between studies. We used the target and land-use intensity as categorical moderators to identify how these factors affected the observed yi and vi (Blüthgen et al. 2012). We also analysed independently the prevalence estimates in vectors and hosts. For pathogens and diseases, we merged similar names for congruency, e.g., we combined "avian Plasmodium" and "avian Haemoproteus" into "avian malaria", since the latter is a more general term used in most studies. We fused several pathogen datasets to increase power of analysis and model strength for groups with very low N, nevertheless we are aware that some of the combined groups might have divergent ecological interactions. Study reference and disease type were included as random factors in both the global model and the host sub-model. In the vector sub-model, disease was excluded because only one type (i.e., human malaria), was found in all three LULC intensity categories, while others only occurred in one or two LULCs, thus not allowing for any comparison. Finally, we used a forest plot (Cuijpers 2016) to detect outliers in the study effects, where data were regarded as homogeneous if the horizontal lines of all studies overlapped (Ried 2006). This was achieved by identifying studies whose 95% CI did not overlap with that of the summary effect. Note that if large studies were outliers, the overall heterogeneity could be high.
Results
What can we learn from the literature search on VBDs?
The literature search returned 3096 unique references about the effect of LULC on VBDs in the first search, and a further 90 articles were added via the bibliographies of the already included articles (including “grey” literature). Of these, 654 articles met our criteria for the systematic literature review (listed in the complete References in the Supplementary Information). However, after reading the title, abstract and keywords, we excluded those articles that did not fulfil the pre-established criteria; thus, the final data set included 162 articles (Table A1), whose full texts were analysed, and 2541 data entries recorded (Fig. 1).
Reviewed articles can be summarized as follows (multiple mentioning of articles possible): 20 studies (12.3%) modelled aspects of any kind of global change and/or prevalence on VBD dynamics (i.e., not showing definite data per LULC or prevalence; Table A2); 11 studies (6.8%) were reviews about aspects of the effect of global change on host, vectors, or parasites (i.e., not providing any additional or new data; Table A3); and 85 studies (52.5%) describe LULC in one of the following ways: agriculture, degraded forest, desert, farmland, forest, savannah, wetland, or included any landscape metric (i.e., not a LULC class but some continuous or disrupted measurement along a different classification of natural, rural, urban, or other environment classification scheme; Table A4). Additionally, 29 studies also discussed LULC according to climate change (Table A5).
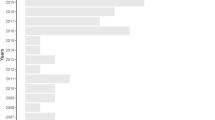
The earliest study we found was published in 1979 (Barrett et al. 1979) and most of the articles (73.5%) were published after 2010. Until the 2000s, the number of articles per year was between one and two, but from 2007 onwards, this rose to an average of around 11 publications reaching the maximum in 2013 and 2016 with 18 publications each (Fig. 2). The first study quantifying prevalence (i.e., human Plasmodium infections in mosquitoes) with qualified but not quantified LULC types was from 2006 (Vittor et al. 2006), while the majority of studies covered the last twenty years (Sebaio et al. 2010; Adja et al. 2011; Laurance et al. 2013; Lüdtke et al. 2013; Alam et al. 2016; Renner et al. 2016; Annetti et al. 2017; Bett et al. 2017; Hernández-Lara et al. 2017; Ferraguti et al. 2018). Globally, we found studies covering 68 countries and territories, although data were unevenly distributed with most records covering parts of North America and Europe (Fig. 3). Indeed, the spatial distribution of data was biased towards a few countries, with the United States the most predominant (15.7%), followed by Spain (8.8%), and Brazil (7.4%). All other countries contributed less than 4.4% each (Table A6).
Cumulative studies per year related to meta-analyses (blue line), review (orange), modelling (grey) and articles with defined land-use and land-cover (LULC) information (yellow). Note: single studies may cover multiple years of publication and may appear in different lines. Reviews are underrepresented due to their exclusion of primary data
Geographic coverage of the 162 studies included in the systematic review. Yellow dots indicate the locations of articles included in the meta-analyses (N = 18). The blue colour scale represents the number of records extracted from different countries, with white areas indicating where no data were found
Within the VBD systems, we found information on 73 different pathogens, causative agents of globally 20 different diseases (Table A7). Avian and human malaria were the two most commonly named diseases and mentioned in 45.9% and 18.9% respectively of the cases in the database, followed by dengue fever (7.2%), West Nile fever (6.5%) and Yellow fever, Japanese fever and Chaoyang fever (4.4% each). All remaining diseases accounted for less than 2% of all entries encountered in the database.
Overall, we found 193 different hosts. The most common were humans (Homo sapiens, 25.5%), followed by house sparrows (Passer domesticus, 13%), while all other hosts contributed less than 9.5% each (Table A8). By contrast, we found 144 different vector groups, predominated by Anopheles (32.9%) and Culex (24.4%) mosquito genera, and all the rest represented less than 13.1% each (Table A9).
Drivers of prevalence of infection outcomes: the meta-analysis
Not all studies on VBDs that were reviewed were suitable for inclusion in the meta-analysis due to a lack of quantified data, discussion on the effects of land-use intensity on infection prevalence, or the necessary information for statistical analysis. Out of the 162 reviewed articles, 18 (11.1%) provided the information needed to perform a meta-analysis, particularly: Andreadis et al. 2008; Adja et al. 2011; Proietti et al. 2011; Laurance et al. 2013; Lüdtke et al. 2013; Andreadis et al. 2014; Alam et al. 2016; Renner et al. 2016; Wilkinson et al. 2016; Annetti et al. 2017; Bett et al. 2017; Hernández-Lara et al. 2017; Ferraguti et al. 2013, 2018; Martínez-de la Puente et al. 2018; Amratia et al. 2019; Jiménez-Peñuela et al. 2019 (see Table 2 for the complete dataset used). It is important to note that our meta-analysis only covers a small subset of all possible LULC types or gradients available on a global scale. Moreover, the studies included in our analysis were conducted under different environmental and temporal contexts, which may have influenced the yi in each study (Barendregt et al. 2013). To account for this heterogeneity, we employed random-effect models to estimate the yi. Our models (Table 3) and forest plot (Fig. 4) showed high heterogeneity in yi across all models. The C.I. provide information on the precision and statistical significance of the included studies. Studies whose C.I. do not intersect the null effect line are considered statistically significant (Fig. 4), particularly Proietti et al. 2011; Laurance et al. 2013; Lüdtke et al. 2013; Renner et al. 2016; Wilkinson et al. 2016; Annetti et al. 2017; Hernández-Lara et al. 2017. However, even if a study's C.I. crosses the null effect line and is not statistically significant, it still provides valuable information, due to its range limits can offer insights into the significance and impact of the study's findings.
Forest plot presenting the results of vector-borne infection prevalence from studies included in the meta-analysis. The plot features the articles included, presenting the effect size of infection (yi) based on the land-use and land-cover (LULC) intensity classification for each study on both sides of the null effect line (dotted line), along with their respective 95% confidence intervals (C.I.) calculated using a random-effects model. The diamond symbol represents the overall outcome of the meta-analysis
Land-use intensity in combination with host and vector targets significantly affects infection prevalence for VBDs (data, yi and vi in Table 2). Indeed, the global multivariate model indicated that host versus vector, in combination with land-use intensity, explained significant variation in infection prevalence outcomes (Table 3). Both tests for residual heterogeneity (QEdf=29 = 12034.894, p < 0.0001) and for moderators (QMdf=3 = 131.692, p < 0.0001) were significant. Analysis of these moderators indicated that vectors and low land-use intensity were associated with lower infection prevalence compared to hosts and high land-use intensity (Table 3). Infection prevalence was also positively related to medium land-use intensity. Results from our alternative meta-analysis based on the HFI further support this outcome (Appendix 3). The significant positive relationship observed between the infection prevalence and the HFI supports our broader findings that land-use intensity significantly affects infection prevalence for VDBs (Table 3, Global Model), regardless of whether HFI values were aligned with the sampling year of each study (Table S1) or corresponded to a single year (Table S2).
Results from the host sub-model supported these general findings, where infection prevalence was significantly lower in low land-use intensity (test for residual heterogeneity: QEdf=26 = 12032.500, p < 0.0001; test of moderators: QMdf=2 = 122.524, p < 0.0001). When focusing solely on vectors, data regarding the three land-use intensity categories were only available for human malaria pathogens, thereby preventing any additional analysis.
No asymmetry was found in the distribution of the values analysed, indicating that there were no outliers in the yi (see Appendix 4 for outlier analyses, Figs. S2–S4). Here, we examined the impact of temporal scale on our results, and our meta-analyses revealed that larger magnitudes of effect sizes (the average values of yi) were well distributed among the different years of the studies (Fig. S3A), thus indicating that outcomes of our study are robust to different temporal scales. Additionally, the yi values extracted from the meta-analysis papers exhibit a temporal progression, with a mean value in 2015, further justifying the use of an LULC map corresponding to this specific time point (Jung et al. 2020). When evaluating publication bias in the selected studies, the funnel plot asymmetry tests for all studies included in the meta-analysis showed higher variance among effects that had larger S.E. and were therefore more susceptible to sampling variability (Fig. S5). However, we did not find any study potentially missing in our global model (i.e., including hosts and vectors); this suggested that there was neither a publication bias in our meta-analyses, nor that any essential study was missing (regression test for funnel plot asymmetry tdf=31 = 5.594, p < 0.01; Appendix 4).
Discussion
While reviewing the impact of LULC on VBDs and how humans in a complex and ever-changing world affect these dynamics, we came across many studies with experimental and modelling evidence, but also a range of reviews on the topic. We found from our literature review that previous research largely focused on modelling the effects of global change on spatial and temporal disease risks or dynamics (Tonnang et al. 2010; Lachish et al. 2011; Loiseau et al. 2012), or elaborated climate change scenarios to assess the likely development of pathogen transmission (Altizer et al. 2006, 2013). However, many studies overlooked important global change drivers such as LULC change (summarized by IPBES/IPCCC 2022).
Changes in LULC can have significant impacts on the composition and diversity of host and vector species, potentially leading to the loss of certain species and the introduction of new ones. This alteration in species composition is closely linked to changes in biodiversity. It is well-established that human activities are associated with biodiversity loss (e.g.,Eglington and Pearce-Higgins 2012; Naeem et al. 2012). Moreover, at the local or regional level, biodiversity loss can either accelerate pathogen transmission rates (Patz et al. 2000) by providing more pathogen reservoirs or decrease transmission by reducing the number of vectors or hosts (Cardinale et al. 2012). For instance, any induced shifts in migration-phenology and species movements (Ishtiaq and Renner 2020) will likely affect pathogen dynamics because migratory species can be sensitive to LULC and land-use intensification (Hickling et al. 2006).
Current predictions suggest that LULC changes can have an impact on VBDs. However, our systematic review has revealed that empirical evidence supporting this association can yield outcomes depending on the specific pathogen system under investigation (Chasar et al. 2009; Loiseau et al. 2010). For instance, in forest ecosystems, the literature has described a response of Haemosporidians to changes in forest management, types, and structure (Chasar et al. 2009; Loiseau et al. 2010; 2012; Lüdtke et al. 2013; van Hoesel et al. 2019, 2020). Moreover, variations between Hawaiian mesic and xeric forests have been shown to explain some prevalence and parasitemia in both avian haemosporidian and avian pox infections (van Riper et al. 1986; 2002). In tropical regions, human-induced changes to the environment can significantly influence the composition of haemosporidian communities (Muriel et al. 2021). Globally, activities such as forest management and fragmentation, urbanization, and agricultural expansion have been associated with outbreaks of various infectious diseases, including Lyme-Borreliosis (Borrelia) in the northeastern United States (LoGiudice et al. 2003), Nipah virus in Malaysia (Chua et al. 1999), and human malaria in regions of the Global South (Vittor et al. 2006; Franklinos et al. 2019). However, the impact of agricultural systems on parasite prevalence, similar to forest systems, remains uncertain due to inconsistent results, with outcomes depending on the specific land-use type, vector, host, and parasite system being studied (Machault et al. 2011; Loiseau et al. 2013; Pérez-Rodríguez et al. 2013b).
Within the context of LULC conversion, deforestation, which is considered one of the greatest threats to biodiversity (Tilman et al. 2017) and human health (Wilkinson et al. 2018), has been predicted to influence disease dynamics (Guégan et al. 2020). However, the number of studies examining the effects of deforestation on VBDs remains limited compared to the global scale of this threat (Sehgal 2010, 2015). In the Amazon region, for example, there is an increasing understanding of the relationship between VBD proliferation and forest destruction, but conflicting results have emerged and remain unresolved (Tucker Lima et al. 2017). Some studies suggest that deforestation increases malaria risk (Vittor et al. 2006, 2009), while others argue the opposite (Valle and Clark 2013), and one study found no clear relationship (Barros and Honorio 2015). Recent research proposes that deforestation may enhance malaria transmission through ecological mechanisms such as creating more breeding habitats for vectors, while simultaneously, malaria can reduce deforestation due to its impact on local economic activity (MacDonald and Mordecai 2019). The ambiguity of these relationships, influenced by the amount of research conducted, highlights the importance of studying VBDs from a One-Health perspective, as the host-vector-parasite dynamics can vary significantly depending on the conservation status of the study areas. It is clear that we are only beginning to grasp the potential impacts of LULC on VBDs and human/wildlife health, emphasizing the need for further research in this field.
While forests have received considerable attention in relation to VBDs and pathogens, other LULC types, such as urban areas, may exhibit altered processes and more complex interactions compared to forested areas (Bradley and Altizer 2007; Bailly et al. 2016; Amratia et al. 2019). We found several studies examining malaria risk in urban environments, often in comparison to rural sites, for both humans and wildlife (Parker et al. 2015; Hernández-Lara et al. 2017; Abella-Medrano et al. 2018; Jiménez-Peñuela et al. 2019). However, to date, no definitive trend regarding the impact of urbanization on malaria risk has emerged. There seems, however, a pattern towards higher infection prevalence in those areas where human land-use is more intense. Finally, our literature review revealed that mosquitoes were the most representative vector, especially the genera Anopheles and Culex that play a crucial role as vectors in the transmission of human and avian malaria, as well as viruses such as West Nile virus (WNV). In fact, they are responsible for more than half of the reports analysed in this review, highlighting the significant role of Culicidae in VBDs (Benelli and Duggan 2018).
Complex responses to land management were also found, with contradicting results (Vittor et al. 2006; Proietti et al. 2011; Laurance et al. 2013; Benelli and Duggan 2018). Agricultural systems, similar to forest systems, have also been predicted to increase parasite prevalence (Machault et al. 2011; Loiseau et al. 2013; Pérez-Rodríguez et al. 2013b). However, the response is still unclear due to lack of consistent results, and it depends on various factors such as the land-use type, vector, host, and parasite system under investigation. For instance, extensive agriculture has been found to reduce the risk or transmission of VBDs (Sarkar et al. 2012). This can be attributed to factors such as the use of pesticides and regular land cultivation, which can decrease the abundance of disease vectors, including mosquitoes (Li et al. 2014). Conversely, an increase of VBDs in extensive agricultural systems was reported, where higher mosquito abundance and increased disease risk was found in areas with extensive rice agriculture (Mwangangi et al. 2010).
Lastly, to summarize the effects of climate change on VBDs, an unclear pattern in which climate change can contract or expand the distribution ranges of disease emerged (Altizer et al. 2013). It is widely acknowledged that accelerated climate warming has already led to changes in the prevalence or incidence of many infectious diseases (Bayoh and Lindsay 2004). The impacts of climate change on VBDs depend on various factors, including habitat, animal community composition, host and vector species, and the specific pathogens involved. Generally, warmer and wetter conditions can accelerate the transmission of certain diseases (Altizer et al. 2013). Climate may influence vector abundance, as higher average temperatures contribute to increased vector spread, higher transmission rates, and greater pathogen prevalence (Ogden and Lindsay 2016). Warmer temperatures can accelerate the larval development of invertebrates while simultaneously reducing the lifespan of adult vectors (Harvell et al. 2002). The relationship between climate/temperature and VBDs has been inferred from the life history of many vectors (Bayoh and Lindsay 2004) and experimentally tested (Bayoh and Lindsay 2004; Palinauskas et al. 2015). Most vectors, such as dipteran insects and ixodid ticks, are exothermal organisms. Although specific details may vary among species and life stages, most vectors thrive within relatively narrow ranges of optimal temperature and humidity. Moreover, there is a direct link between the incubation temperature of parasites inside vectors and the development of parasites, such as Haemosporidians, which develop optimally under specific vector temperature conditions (Palinauskas et al. 2015).
Factors including spatial and temporal dynamics, data availability and quality, as well as scale and resolution, contribute to the intricacies involved in studying the effects of land-use changes on VBDs. These complexities become evident when considering land-use change, which has significantly accelerated in numerous regions worldwide, particularly in forested areas (FAO 2015; Winkler et al. 2021). Additionally, the impacts of land-use changes on VBDs can differ at local, regional, and global scales. For instance, LULC exhibits regional dominance with different areas experiencing varying degrees of tropical deforestation, agricultural expansion, temperate reforestation, afforestation, cropland intensification, and urbanization (Song et al. 2018). Across all climate domains, montane systems have gained tree cover, while many arid and semi-arid ecosystems have lost vegetation cover (Song et al. 2018). Choosing an appropriate scale for analysis is therefore crucial to capture the relevant processes and patterns, but it can add complexity due to the need for spatially explicit data and the challenges of scaling up or down findings.
Meta-analysis lessons
Our meta-analyses revealed a significant relationship between land-use intensity, or human landscape alteration, and the prevalence of VBDs, depending on whether we considered the host, vector, or both targets. These findings suggest that LULC play a crucial role in determining the prevalence of infection in both vertebrate hosts and insect vectors (Chasar el al. 2009). Specifically, we observed that lower land-use intensity and vectors were associated with lower prevalence of VBDs, whereas medium land-use intensity was linked to higher prevalence. Additionally, higher HFI values facilitate the prevalence of vector-borne diseases in both vector and host populations. These findings strengthen the reliability of the categorical classification system used, further supporting that the most pronounced effects on VBD prevalence occur during the early stages of land use degradation. For instance, fragmented forest areas exhibit high malaria prevalence due to the increased availability of breeding sites for vectors and accelerated life cycles resulting from elevated temperatures in these regions (Laurance et al 2013). Both meta-analyses further supported these general outcomes. Similarly, effects of land use on hosts have been observed in recent WNV outbreaks in Europe, where land use, particularly in agriculture and rural environments classified as medium land-use intensity in this study, has been identified as a key factor (Giesen et al. 2023). It is important to note that the land-use intensity categories used in our study are simplifications and may not fully capture the complexity of anthropogenic actions. Nonetheless, despite our relatively straightforward classification, our findings emphasize the significance of considering land-use intensity when investigating the prevalence of VBDs. Future studies should aim to use continuous measures (e.g., Beyer et al 2020) or provide comprehensive definitions for any land-use categories employed.
Three main outcomes are therefore essential to consider while interpreting the result on VBDs: (i) vector-borne diseases are responding to land-use intensification; (ii) while many more vector taxa were included, the number of studies for vector sub-models was low compared to studies working on hosts and we should either provide more evidence in field/laboratory studies or continue to rely on modelling approaches. However, the test for heterogeneity indicated that differences between studies were explained by moderators included in the model (host or vector combined with LULC intensity) and were therefore not random. Lastly, (iii) the test for moderators showed that both LULC intensity together with host and vectors affected the prevalence of infection.
Limitations of the study
Our literature review predominantly focuses on the last two decades, with a scarcity of studies from earlier periods. This poses a challenge as older studies could have provided insights into long-term changes, but their limited availability hinders their analytical relevance for our study. However, LULC data used in our study were valid to assess land-use type and intensity for the study period considered. As an example, deforestation and land-cover change in Asia vary across specific countries, with some exhibiting a slowing down while others experiencing increased speed over time (Estoque et al. 2019; Suarez-Rubio et al. 2020). Such complexities highlight the need to assess the spatial and temporal dynamics of forest cover (LULC in general) in different regions and to determine its effects of VBD dynamics. Understanding these dynamics requires detailed data on land-use patterns and disease occurrence over time, although collecting such data can be challenging, particularly in large regions or areas with limited resources. Similarly, disease surveillance data in regions with limited monitoring systems or data collection efforts may be incomplete or biased, leading to uncertainties in the analysis.
Additionally, our study was subject to certain limitations due to insufficient or incomplete information on the status of LULC, or gaps, in the coverage of studies on pathogens, vectors and their hosts. Accurate and reliable data on land-use changes and VBDs are crucial for studying their relationship. However, obtaining comprehensive and up-to-date data on land-use changes can be challenging as LULC has varying impacts on the mechanisms of VBD transmission. For instance, habitat fragmentation is often associated with an increase in tick-borne diseases (Lauterbach et al. 2013), whereas deforestation may affect the intensity of mosquito-borne diseases (Franklinos et al. 2019). To account for these impacts, we attempted to distinguish between different impacts of LULC intensity on the prevalence in the host and/or vectors. However, the information available was not always extractable or reported to be able to include it in a meta-analysis. Indeed, one of the largest persisting challenges in performing a meta-analysis is identifying appropriate studies that can be included and that report required data, such as the prevalence of infections and correction-measures such as S.D., used in analyses to estimate the precision of the study results and to calculate the overall yi. Unfortunately, only some of the studies included the necessary data to calculate the prevalence and S.D. Furthermore, the variability in the way that studies are conducted and reported can also make it challenging to compare results across studies. For example, different studies may use different methods for diagnosing infections, different sampling techniques, or different definitions of an infection. These differences can introduce bias or confounding variables into the meta-analysis, which make it more difficult to obtain accurate estimates of the overall yi.
Open data and data gaps
The limited availability of "open data" currently restricted the dataset for our meta-analysis. This information is vital to fully understand the direction and magnitude of the effects of land-use intensification on VBDs. To achieve this goal, several research gaps need to be addressed: first, studies should report easily extractable and reproducible indicators on sampling effort (N), infection prevalence in host and/or vector and, most importantly, information on the variability or dispersion of a set of values, such as S.D., S.E., and/or 95% CI (open data approach, as propagated, for example, by the European Union). Second, studies should accurately describe and/or classify LULC to match the intensity of land-use change. Addressing these gaps entails obtaining more comprehensive empirical data to understand the impact of land-use changes on VBDs. Therefore, future studies should incorporate information on LULC to determine the impact on the transmission of VBD, and all conclusions should be considered in the overall context of the interacting effects of global change, as well as the interaction of hosts, vectors, and their pathogens.
Future research directions
Future studies should incorporate the independent and interactive effects of land-use intensity into VBD analyses in natural and human-dominated ecosystems in order to fill some persistent knowledge gaps for the disease with relevance to humans and wildlife. These efforts should aim to cover most climate zones, but with a specific focus on the tropics since: (1) to date, few LULC studies have focused on these, and (2) tropical forests undergoing land-use change are global hotspots for outbreaks of emerging infectious diseases and are postulated to be reservoirs for other infectious diseases. Additionally, if possible, studies should include modelling approaches combined with experimental manipulation. All studies must comply with a strict “open data” policy (but considering all privacy issues if human data are involved) to maximize the availability of evidence for future studies. At least the latter will greatly diminish a current impediment to some meta-analytic studies.
By identifying opportunities to mitigate LULC driven disease emergence, we will be better prepared to face the future challenges of VBDs, which will impact global health systems and ultimately the worldwide economy. Understanding the different effects of land-use intensity on VBDs is relevant for the implementation of specific management strategies, according to vector or host habitat types. Thus, LULC should be included in strategies to control malaria, chikungunya virus, Lyme-borreliosis, and other VBDs, all of which can have significant negative implications for humans, livestock and wildlife (Kilpatrick and Randolph 2012; Stanaway et al. 2016; WHO and UNICEF 2017; Rohr et al. 2020).
While the impact of land-use change on VBD risk has been shown to be significant (Sehgal 2010, 2015; Lüdtke et al. 2013; Renner et al. 2016), it often receives less attention in discussions due to the focus on more recent timescales and the presumed immediate effects of other global change processes and their interactions on VBDs (Sala et al. 2000; Reid et al. 2005). While this may be true for the effects of climate change on vectors (i.e., temperature affects dipteran vector development), LULC is likely to affect hosts (van Hoesel et al. 2019, 2020) (i.e., resource availability is altered) in a more direct and imminent way.
Data availability
All data are available from the Supplementary Information and the reference list. All relevant data were included in the paper.
References
Abella-Medrano CA, Ibanez-Bernal S, Carbo-Ramirez P, Santiago-Alarcon D (2018) Blood-meal preferences and avian malaria detection in mosquitoes (Diptera: Culicidae) captured at different land use types within a neotropical montane cloud forest matrix. Parasitol Int 67(3):313–320
Adja AM, Ngoran EK, Koudou BG et al (2011) Contribution of Anopheles funestus, An. gambiae and An. nili (Diptera: Culicidae) to the perennial malaria transmission in the southern and western forest areas of Cote d’Ivoire. Ann Trop Med Parasitol 105(1):13–24
Alam MS, Kabir MM, Hossain MS et al (2016) Reduction in malaria prevalence and increase in malaria awareness in endemic districts of Bangladesh. Malar J 15(1):552
Alimi Y, Bernstein A, Epstein J et al (2021) Report of the scientific task force on preventing pandemics. Harvard Chan C-CHANGE and Harvard Global Health Institute, Cambridge
Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9(4):467–484
Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD (2013) Climate change and infectious diseases: from evidence to a predictive framework. Science 341(6145):514–519
Amratia P, Psychas P, Abuaku B et al (2019) Characterizing local-scale heterogeneity of malaria risk: a case study in Bunkpurugu-Yunyoo district in northern Ghana. Malar J 18(1):81
Andreadis TG, Anderson JF, Armstrong PM, Main AJ (2008) Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: a ten-year analysis, 1997–2006. Vector Borne Zoonotic Dis 8(2):175–188
Andreadis TG, Armstrong PM, Anderson JF, Main AJ (2014) Spatial-temporal analysis of Cache Valley virus (Bunyaviridae: Orthobunyavirus) infection in anopheline and culicine mosquitoes (Diptera: Culicidae) in the northeastern United States, 1997–2012. Vector Borne Zoonotic Dis 14(10):763–773
Annetti KL, Rivera NA, Andrews JE, Mateus-Pinilla N (2017) Survey of Haemosporidian parasites in resident and migrant game birds of Illinois. J Fish Wildl Manag 8(2):661–668
Bailly J, Scheifler R, Belvalette M et al (2016) Negative impact of urban habitat on immunity in the great tit Parus major. Oecologia 182(4):1053–1062
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T (2013) Meta-analysis of prevalence. J Epidemiol Commun Health 67(11):974–978
Barrett TV, Hoff R, Mott KE, Guedes F, Sherlock IA (1979) An outbreak of acute Chagas’s disease in the São Francisco Valley region of Bahia, Brazil: triatomine vectors and animal reservoirs of Trypanosoma cruzi. Trans R Soc Trop Med Hyg 73(6):703–709
Barros FS, Honorio NA (2015) Deforestation and malaria on the Amazon frontier: Larval clustering of Anopheles darlingi (Diptera: Culicidae) determines focal distribution of malaria. Am J Trop Med Hyg 93(5):939–953
Bayoh MN, Lindsay SW (2004) Temperature-related duration of aquatic stages of the Afrotropical malaria vector mosquito Anopheles gambiae in the laboratory. Med Vet Entomol 18(2):174–179
Beaute J, Spiteri G, Warns-Petit E, Zeller H (2018) Tick-borne encephalitis in Europe, 2012 to 2016. Euro Surveill 23(45):1800201
Benelli G, Duggan MF (2018) Management of arthropod vector data–Social and ecological dynamics facing the One Health perspective. Acta Trop 182:80–91
Bett B, Said MY, Sang R et al (2017) Effects of flood irrigation on the risk of selected zoonotic pathogens in an arid and semi-arid area in the eastern Kenya. PLoS ONE 12(5):e0172626
Beyer HL, Venter O, Grantham HS, Watson JE (2020) Substantial losses in ecoregion intactness highlight urgency of globally coordinated action. Conserv Lett 13(2):e12692
Blüthgen N, Dormann CF, Prati D et al (2012) A quantitative index of land-use intensity in grasslands: Integrating mowing, grazing and fertilization. Basic Appl Ecol 13(3):207–220
Bradley CA, Altizer S (2007) Urbanization and the ecology of wildlife diseases. Trends Ecol Evol 22(2):95–102
Caminade C, McIntyre KM, Jones AE (2019) Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci 1436(1):157–173
Cardinale BJ, Duffy JE, Gonzalez A et al (2012) Biodiversity loss and its impact on humanity. Nature 486(7401):59–67
Chasar A, Loiseau C, Valkiunas G, Iezhova T, Smith TB, Sehgal RN (2009) Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol Ecol 18(19):4121–4133
Chase JM, Blowes SA, Knight TM, Gerstner K, May F (2020) Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 584(7820):238–243
Chua KB, Goh KJ, Wong KT et al (1999) Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. The Lancet 354(9186):1257–1259
Cuijpers P (2016) Meta-analyses in mental health research. A practical guide. Pim Cuijpers Uitgeverij, Amsterdam
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Eglington SM, Pearce-Higgins JW (2012) Disentangling the relative importance of changes in climate and land-use intensity in driving recent bird population trends. PLoS ONE 7(3):e30407
Ergönül Ö (2006) Crimean-Congo haemorrhagic fever. Lancet Infect Dis 6(4):203–214
Estoque RC, Ooba M, Avitabile V et al (2019) The future of Southeast Asia’s forests. Nat Commun 10(1):1829
FAO (2015) Global Forest Resources Assessment 2015. FAO Forestry Paper No. 1. UN Food and Agriculture Organization, Rome.
Ferraguti M, Martínez-de la Puente J, Bensch S et al (2018) Ecological determinants of avian malaria infections: an integrative analysis at landscape, mosquito and vertebrate community levels. J Anim Ecol 87(3):727–740
Ferraguti M, Martínez-de la Puente J, Munoz J et al (2013) Avian Plasmodium in Culex and Ochlerotatus mosquitoes from southern Spain: effects of season and host-feeding source on parasite dynamics. PLoS ONE 8(6):e66237
Franklinos LHV, Jones KE, Redding DW, Abubakar I (2019) The effect of global change on mosquito-borne disease. Lancet Infect Dis 19(9):e302–e312
Galbraith RF (1994) Some applications of radial plots. J Am Stat Assoc 89(428):1232–1242
Gilbert L (2021) The impacts of climate change on ticks and tick-borne disease risk. Annu Rev Entomol 66:373–388
Guégan J-F, Ayouba A, Cappelle J, De Thoisy B (2020) Forests and emerging infectious diseases: unleashing the beast within. Environ Res Lett 15(8):083007
Giesen C, Herrador Z, Fernandez B, Figuerola J, Gangoso L, Vazquez A, Gómez-Barroso D (2023) A systematic review of environmental factors related to WNV circulation in European and Mediterranean countries. One Health 16:100478
Harvell CD, Mitchell CE, Ward JR et al (2002) Climate warming and disease risks for terrestrial and marine biota. Science 296(5576):2158–2162
Hernández-Lara C, González-García F, Santiago-Alarcon D (2017) Spatial and seasonal variation of avian malaria infections in five different land use types within a Neotropical montane forest matrix. Landsc Urban Plan 157:151–160
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Change Biol 12(3):450–455
Ishtiaq F, Renner SC (2020) Bird migration and vector-borne parasite transmission. In: Santiago AD, andMarzal A. (eds) Avian malaria and related parasites in the tropics. Springer, New York
Jiménez-Peñuela J, Ferraguti M, Martínez-de la Puente J, Soriguer R, Figuerola J (2019) Urbanization and blood parasite infections affect the body condition of wild birds. The Science of the Total Environment 651(Pt 2):3015–3022
Jovani R, Tella JL (2006) Parasite prevalence and sample size: misconceptions and solutions. Trends Parasitol 22:214–218
Jung M, Dahal PR, Butchart SHM et al (2020) A global map of terrestrial habitat types. Sci Data 7(1):256
Kilpatrick AM, Randolph SE (2012) Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380(9857):1946–1955
Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon BC (2011) Infection dynamics of endemic malaria in a wild bird population: parasite species-dependent drivers of spatial and temporal variation in transmission rates. J Anim Ecol 80(6):1207–1216
Laurance SGW, Jones D, Westcott D, McKeown A, Harrington G, Hilbert DW (2013) Habitat fragmentation and ecological traits influence the prevalence of avian blood parasites in a tropical rainforest landscape. PLoS ONE 8(10):48
Lauterbach R, Wells K, O’Hara RB, Kalko EK, Renner SC (2013) Variable strength of forest stand attributes and weather conditions on the questing activity of Ixodes ricinus ticks over years in managed forests. PLoS ONE 8(1):e55365
Li Y, Kamara F, Zhou G et al (2014) Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl Trop Dis 8(11):e3301
LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F (2003) The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA 100(2):567–571
Loiseau C, Harrigan RJ, Bichet C et al (2013) Predictions of avian Plasmodium expansion under climate change. Sci Rep 3:1126
Loiseau C, Harrigan RJ, Robert A et al (2012) Host and habitat specialization of avian malaria in Africa. Mol Ecol 21(2):431–441
Loiseau C, Iezhova T, Valkiunas G et al (2010) Spatial variation of haemosporidian parasite infection in African rainforest bird species. J Parasitol 96(1):21–29
Lüdtke B, Moser I, Santiago-Alarcon D et al (2013) Associations of forest type, parasitism and body condition of two European passerines, Fringilla coelebs and Sylvia atricapilla. PLoS ONE 8(12):e81395
MacDonald AJ, Mordecai EA (2019) Amazon deforestation drives malaria transmission, and malaria burden reduces forest clearing. Proc Natl Acad Sci USA 116(44):22212–22218
Machault V, Vignolles C, Borchi F et al (2011) The use of remotely sensed environmental data in the study of malaria. Geospat Health 5(2):151–168
Martínez-de la Puente J, Ferraguti M, Ruiz S et al (2018) Mosquito community influences West Nile virus seroprevalence in wild birds: implications for the risk of spillover into human populations. Sci Rep 8(1):1–7
Mu H, Li X, Wen Y et al (2022) A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci Data 9(1):176
Muriel J, Marzal A, Magallanes S et al (2021) Prevalence and diversity of avian haemosporidians increase with anthropogenic disturbance in tropical habitats in Myanmar. Diversity 13(3):1–19
Mwangangi JM, Shililu J, Muturi EJ et al (2010) Anopheles larval abundance and diversity in three rice agro-village complexes Mwea irrigation scheme, central Kenya. Malar J 9(1):1–10
Naeem S, Duffy JE, Zavaleta E (2012) The functions of biological diversity in an age of extinction. Science 336(6087):1401–1406
Ogden NH, Lindsay LR (2016) Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol 32(8):646–656
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10(1):1–11
Palinauskas V, Ziegyte R, Ilgunas M et al (2015) Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int J Parasitol 45(1):51–62
Parker DM, Carrara VI, Pukrittayakamee S, McGready R, Nosten FH (2015) Malaria ecology along the Thailand-Myanmar border. Malar J 14:388
Patz JA, Graczyk TK, Geller N, Vittor AY (2000) Effects of environmental change on emerging parasitic diseases. Int J Parasitol 30(12–13):1395–1405
Pérez-Rodríguez A, de la Puente J, Onrubia A, Perez-Tris J (2013a) Molecular characterization of haemosporidian parasites from kites of the genus Milvus (Aves: Accipitridae). Int J Parasitol 43(5):381–387
Pérez-Rodríguez A, Fernandez-Gonzalez S, de la Hera I, Perez-Tris J (2013b) Finding the appropriate variables to model the distribution of vector-borne parasites with different environmental preferences: climate is not enough. Glob Chang Biol 19(11):3245–3253
Proietti C, Pettinato DD, Kanoi BN et al (2011) Continuing intense malaria transmission in northern Uganda. Am J Trop Med Hyg 84(5):830–837
Reid WV, Mooney HA, Cropper A et al (2005) Ecosystems and human well-being-synthesis: a report of the millennium ecosystem assessment. Island Press, Washington
Renner SC, Lüdtke B, Kaiser S et al (2016) Forests of opportunities and mischief: disentangling the interactions between forests, parasites and immune responses. Int J Parasitol 46(9):571–579
Ried K (2006) Interpreting and understanding meta-analysis graphs: a practical guide. Aust Fam Physician 35(8):635–638
Rohr JR, Civitello DJ, Halliday FW et al (2020) Towards common ground in the biodiversity-disease debate. Nat Ecol Evol 4(1):24–33
Rosenberg R, Lindsey NP, Fischer M et al (2018) Vital signs: trends in reported vectorborne disease cases—United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67(17):496–501
Sala OE, Chapin FS 3rd, Armesto JJ et al (2000) Global biodiversity scenarios for the year 2100. Science 287(5459):1770–1774
Sarkar A, Aronson KJ, Patil S, Hugar LB (2012) Emerging health risks associated with modern agriculture practices: a comprehensive study in India. Environ Res 115:37–50
Sebaio F, Braga ÉM, Branquinho F, Manica LT, Marini MÂ (2010) Blood parasites in Brazilian Atlantic Forest birds: effects of fragment size and habitat dependency. Bird Conserv Int 20(4):432–439
Sehgal RN (2010) Deforestation and avian infectious diseases. J Exp Biol 213(6):955–960
Sehgal RN (2015) Manifold habitat effects on the prevalence and diversity of avian blood parasites. Int J Parasitol Parasites Wildl 4(3):421–430
Song XP, Hansen MC, Stehman SV et al (2018) Global land change from 1982 to 2016. Nature 560(7720):639–643
Stanaway JD, Shepard DS, Undurraga EA et al (2016) The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 16(6):712–723
Suarez-Rubio M, Connette G, Aung; T, Kyaw; M, Renner SC, (2020) Hkakabo Razi Landscape as one of the last exemplar of large contiguous forests. Sci Rep 10(1):14005
Tilman D, Clark M, Williams DR, Kimmel K, Polasky S, Packer C (2017) Future threats to biodiversity and pathways to their prevention. Nature 546(7656):73–81
Tolle MA (2009) Mosquito-borne diseases. Curr Probl Pediatr Adolesc Health Care 39(4):97–140
Tonnang HE, Kangalawe RY, Yanda PZ (2010) Predicting and mapping malaria under climate change scenarios: the potential redistribution of malaria vectors in Africa. Malar J 9:111
Tucker Lima JM, Vittor A, Rifai S, Valle D (2017) Does deforestation promote or inhibit malaria transmission in the Amazon? A systematic literature review and critical appraisal of current evidence. Philos Trans R Soc B 372(1722):20160125
Valle D, Clark J (2013) Conservation efforts may increase malaria burden in the Brazilian Amazon. PLoS ONE 8(3):e57519
van Hoesel W, Marzal A, Magallanes S, Santiago-Alarcon D, Ibanez-Bernal S, Renner SC (2019) Management of ecosystems alters vector dynamics and haemosporidian infections. Sci Rep 9(1):8779
van Hoesel W, Santiago Alarcon D, Marzal A, Renner SC (2020) Effects of forest structure on the interaction between avian hosts, dipteran vectors and haemosporidian parasites. BMC Ecol 20(1):47
van Riper C, van Riper SG, Goff ML, Laird M (1986) The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecol Monogr 56(4):327–344
van Riper C, van Riper SG, Hansen WR (2002) Epizootiology and effect of avian pox on Hawaiian forest birds. Auk 119(4):929–942
Vittor AY, Gilman RH, Tielsch J et al (2006) The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg 74(1):3–11
Vittor AY, Pan W, Gilman RH et al (2009) Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg 81(1):5–12
WHO, UNICEF (2017) Global vector control response 2017–2030.
Wilkinson DA, Marshall JC, French NP, Hayman DTS (2018) Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J R Soc Interface 15(149):158
Wilkinson LC, Handel CM, Van Hemert C, Loiseau C, Sehgal RN (2016) Avian malaria in a boreal resident species: long-term temporal variability, and increased prevalence in birds with avian keratin disorder. Int J Parasitol 46(4):281–290
Winkler K, Fuchs R, Rounsevell M, Herold M (2021) Global land use changes are four times greater than previously estimated. Nat Commun 12(1):2501
Acknowledgements
We would like to thank Carlos Mora Rubio for his help in selecting some articles to review. We thank Yolanda Wiersma and two anonymous reviewers for valuable comments on a previous version of the manuscript. MF was supported by a Juan de la Cierva 2017 Formación contract (FJCI-2017-34394) from the Ministry of Science, Innovation and Universities, and she is currently financed by a Ramón y Cajal postdoctoral contract (RYC2021-031613-I) from the Spanish Ministry of Science and Innovation (MICINN). SCR was supported by DFG Re1733/6-1 for part of this work.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
SCR developed the concept and design of the study. MF, SM, and SCR searched, read, screened, selected the articles, extracted the information, and performed all statistical analysis. SM extrapolated the land-use and land-cover intensity data, the Human Footprint Index, and performed the meta-analysis. The first draft of the manuscript was written by SCR, MSR, AM and PJJB. MF and SM equally contributed to this article. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding authors
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferraguti, M., Magallanes, S., Suarez-Rubio, M. et al. Does land-use and land cover affect vector-borne diseases? A systematic review and meta-analysis. Landsc Ecol 38, 2433–2451 (2023). https://doi.org/10.1007/s10980-023-01746-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-023-01746-3